VTE can be considered as a multicausal disease involving various inherited and acquired prothrombotic conditions. Although greater emphasis has classically been given to traditional thrombophilic risk factors, there is increasing recognition of less typical precipitating conditions and events. In this article, the authors focus on the most promising, persistent, and potentially treatable novel risk factors.
Venous thromboembolism (VTE), such as deep venous thrombosis (DVT) of the leg, pulmonary embolism (PE), and unusual-site thrombosis, is a common disorder. Vein thrombus either arises spontaneously or is caused by well-known clinical conditions. Orthopedic and cancer surgery, trauma and spinal cord injury, metastatic cancer, and acute medical illness are the most common settings in which thromboembolism occurs. Besides major risk factors of VTE, several genetic and acquired minor risks have been identified. Indeed, VTE is currently best understood as a multicausal disease in which more than one genetic or environmental condition coincides to produce clinically apparent thrombosis. Therefore, weak thromboembolic risk factors may also be clinically relevant, especially if treatable. Novel risk factors for VTE, which were previously not described, are frequently reported. This article discusses the most promising risk factors for VTE: traditional cardiovascular risk factors, JAK2 mutation, and endocrine disorders ( Box 1 ).
Cardiovascular risk factors
- •
Hypertension
- •
Obesity
- •
Diabetes mellitus
- •
Dyslipidemia
- •
Smoking
- •
Metabolic syndrome
Ph− myeloproliferative disorders and JAK2V617F mutation
Endocrine disorders
- •
Thyroid dysfunction: hyperthyroidism and subclinical hypothyroidism
- •
Cushing syndrome
- •
Hyperprolactinemia
- •
Traditional cardiovascular risk factors
Arterial atherothrombotic disease and VTE were generally considered as separate entities from mechanistic and clinical points of view. However, several studies have recently challenged this dichotomy, suggesting a closer link between these 2 clinical conditions.
Among traditional cardiovascular risk factors, only obesity and age have consistently been demonstrated to be independent risk factors for VTE. In a prospective cohort study on 855 men, waist circumference greater than 100 cm was found to be associated with VTE (odds ratio [OR], 3.92; 95% confidence interval [CI], 2.10–7.29). These results were confirmed by a similar study performed in 112,822 women in whom obesity was an independent predictor of PE and by a population-based study in which obesity was strongly associated with the risk of DVT in men and women.
Recently, several observational studies have also reported a positive association between dyslipidemia and VTE. Elevated levels of triglycerides and low levels of high-density lipoprotein (HDL) were found to increase the risk of VTE, whereas increased HDL levels may protect against VTE. Doggen and colleagues found that elevated serum triglyceride levels are associated with a doubling of the risk of VTE in postmenopausal women. Another study identified a link between increased levels of lipoprotein (a), a marker of atherosclerosis, and the risk of unprovoked VTE (OR, 2.1; 95% CI, 1.4–3.2). Observational studies have also reported a positive association between DVT and/or PE and diabetes, arterial hypertension, and smoking.
However, other studies have failed to find a significant association between these traditional cardiovascular risk factors and VTE. For example, in a study on 19,293 subjects, a higher incidence of VTE was associated with obesity (OR, 2.27; 95% CI, 1.57–3.28) and diabetes (OR, 1.7; 95% CI, 1.2–2.4) but not with hypertension at 8 years of follow-up, and in another study that enrolled 18,662 male physicians, VTE was associated with obesity but not with hypertension, hypercholesterolemia, diabetes, or smoking at 20 years of follow-up.
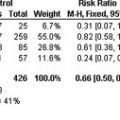
To further assess the strength of the evidence supporting the association between cardiovascular risk factors and VTE, Ageno and colleagues recently performed a systematic review and a meta-analysis of the literature. The prevalence of 5 major established risk factors for atherosclerosis (obesity, arterial hypertension, diabetes mellitus, smoking, and dyslipidemia) was compared in patients with VTE and in controls based on data from 21 selected case-control and cohort studies, including a total of more than 60,000 patients and controls. Obesity (OR, 2.33; 95% CI, 1.68–3.24), hypertension (OR, 1.51; 95% CI, 1.23–1.85), and diabetes mellitus (OR, 1.42; 95% CI, 1.12–1.77) were associated with VTE. There was no significant association with smoking (OR, 1.18; 95% CI, 0.95–1.46). Hypercholesterolemia was not associated with an increased risk of VTE (OR, 1.16; 95% CI, 0.67–2.02), but the weighted mean in HDL cholesterol levels was significantly lower in patients with VTE than in controls (−2.86 mg/dL; 95% CI, −4.34 to −1.38, P <.05). Likewise, triglyceride levels were, on an average, 21.0 mg/dL (95% CI, 10.0–31.0) higher in patients with VTE than in controls. No significant difference was observed for low-density lipoprotein cholesterol levels in both the groups, although the small number of included studies did not allow any meaningful conclusion.
Although these results should be considered with caution because of the design of included studies and because of the significant heterogeneity among the studies for all variables apart from diabetes, there is reasonable biologic plausibility underlying the hypothesis that VTE is associated with risk factors for cardiovascular disease. Obesity and diabetes are known to predispose patients to hypercoagulable and inflammatory states, hypertension may induce endothelial dysfunction, and dyslipidemia is also associated with hypercoagulability and endothelial dysfunction. All such effects, in particular when combined, may induce a prothrombotic effect that could also predispose patients to an increased risk of VTE. This association might help explain, in part, the pathogenesis of many idiopathic episodes of VTE.
Recent studies have addressed the relationship between VTE and the metabolic syndrome, which is a cluster of cardiovascular risk factors, including obesity, hyperlipidemia, hypertension, and hyperglycemia. Ageno and colleagues observed a significantly higher prevalence of this syndrome among patients with idiopathic DVT than among matched controls without venous thrombosis. These findings were confirmed by the results of 2 other case-control studies in European and Korean patients.
Although recent trials have suggested that drugs traditionally used for primary and secondary prevention of atherosclerotic disease (such as statins) may also reduce the incidence of VTE, further studies are warranted to confirm these preliminary findings.
Philadelphia-negative myeloproliferative neoplasms and JAK2 mutation
Splanchnic vein thromboses (SVTs), which include portal vein thrombosis, mesenteric vein thrombosis, and thrombosis of the hepatic veins (causing Budd-Chiari syndrome), are frequently the first manifestations of Philadelphia-negative (Ph−) myeloproliferative neoplasms (MPNs), polycythemia vera (PV), and essential thrombocythemia (ET). Overall, Ph− MPNs are among the most common causes of Budd-Chiari syndrome and portal vein thrombosis, being found in up to 50% and 25% of the patients, respectively. The identification of occult MPNs in patients with VTE has traditionally been based on bone marrow biopsy findings and endogenous erythroid colony formation assessment. The discovery of the gain-of-function JAK2V617F mutation, found in approximately 90% of patients with PV and in 50% of those with ET or primary myelofibrosis (PM), has modified the diagnostic approach to Ph− MPNs.
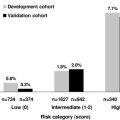
Several studies and a recent meta-analysis have explored whether screening for the JAK2V617F mutation in patients presenting with venous thrombosis without overt MPN is justified. The mean prevalence of the JAK2 mutation was 32.7% (95% CI, 25.5%–35.9%) in patients with SVT; the mutation was associated with an increased risk of SVT (OR, 53.98; 95% CI, 13.10–222.45) and with a subsequent diagnosis of MPNs in many of these patients. The JAK2 mutation was rare in patients with VTE in other locations (frequency, 0.88% to 2.57%). These results suggest that screening for the JAK2 mutation be considered in patients with SVT because this mutation is a strong predictor of a subsequent development of an MPN. A recent meta-analysis comparing patients with and without the mutation explored the mutation’s possible role as a procoagulant in ET and in PM. In patients with ET, JAK2V617F mutation was associated with a significant increased risk of thrombosis (OR, 1.92; 95% CI, 1.45–2.53), both of venous (OR, 2.49; 95% CI, 1.71–3.61) and arterial (OR, 1.77; 95% CI, 1.29–2.43) vessels. In patients with PM, the presence of JAK2V617F mutation was associated with a trend toward increased risk of thrombosis (OR, 1.76; 95% CI, 0.91–3.41). The recommendation for screening is strengthened by the observation that the concomitant presence of portal hypertension and hypersplenism could mask the increase in blood cell counts, which is the cardinal manifestation of the MPNs.
Why the JAK2 mutation is associated with SVT is unknown. The mutation causes constitutive activation of JAK2 , which in turn results in cytokine-independent myeloproliferation, mobilization of blood cell progenitors, and spontaneous formation of endogenous erythroid colonies leading to the development of the MPN. How these factors predict for splanchnic but not other forms of VTE is unknown.
Philadelphia-negative myeloproliferative neoplasms and JAK2 mutation
Splanchnic vein thromboses (SVTs), which include portal vein thrombosis, mesenteric vein thrombosis, and thrombosis of the hepatic veins (causing Budd-Chiari syndrome), are frequently the first manifestations of Philadelphia-negative (Ph−) myeloproliferative neoplasms (MPNs), polycythemia vera (PV), and essential thrombocythemia (ET). Overall, Ph− MPNs are among the most common causes of Budd-Chiari syndrome and portal vein thrombosis, being found in up to 50% and 25% of the patients, respectively. The identification of occult MPNs in patients with VTE has traditionally been based on bone marrow biopsy findings and endogenous erythroid colony formation assessment. The discovery of the gain-of-function JAK2V617F mutation, found in approximately 90% of patients with PV and in 50% of those with ET or primary myelofibrosis (PM), has modified the diagnostic approach to Ph− MPNs.
Several studies and a recent meta-analysis have explored whether screening for the JAK2V617F mutation in patients presenting with venous thrombosis without overt MPN is justified. The mean prevalence of the JAK2 mutation was 32.7% (95% CI, 25.5%–35.9%) in patients with SVT; the mutation was associated with an increased risk of SVT (OR, 53.98; 95% CI, 13.10–222.45) and with a subsequent diagnosis of MPNs in many of these patients. The JAK2 mutation was rare in patients with VTE in other locations (frequency, 0.88% to 2.57%). These results suggest that screening for the JAK2 mutation be considered in patients with SVT because this mutation is a strong predictor of a subsequent development of an MPN. A recent meta-analysis comparing patients with and without the mutation explored the mutation’s possible role as a procoagulant in ET and in PM. In patients with ET, JAK2V617F mutation was associated with a significant increased risk of thrombosis (OR, 1.92; 95% CI, 1.45–2.53), both of venous (OR, 2.49; 95% CI, 1.71–3.61) and arterial (OR, 1.77; 95% CI, 1.29–2.43) vessels. In patients with PM, the presence of JAK2V617F mutation was associated with a trend toward increased risk of thrombosis (OR, 1.76; 95% CI, 0.91–3.41). The recommendation for screening is strengthened by the observation that the concomitant presence of portal hypertension and hypersplenism could mask the increase in blood cell counts, which is the cardinal manifestation of the MPNs.
Why the JAK2 mutation is associated with SVT is unknown. The mutation causes constitutive activation of JAK2 , which in turn results in cytokine-independent myeloproliferation, mobilization of blood cell progenitors, and spontaneous formation of endogenous erythroid colonies leading to the development of the MPN. How these factors predict for splanchnic but not other forms of VTE is unknown.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree