Fig. 3.1
Biomarkers have to be very prognostic for the specific physiopathological conditions inherent with the pathology. Being the chronicity of inflammation, the base for the genesis and the progression of chronic-degenerative pathologies, as tumors, the prognostic biomarkers have to be informative of a few points in order to be suitable in the prevention and treatment of these points. These pathologies include (a) the normal healthy state, (b) the transient inflammatory state, and (c) the chronic inflammatory state. The reason of these considerations is that the significance of these biomarkers depend on their prognostic capacity for the identification of the passages (1) from the healthy condition, a state of homeostasis, whose biomarker is defined as the a type and it is index of no risk of pathology, (2) to the transient inflammation, a state in which the chance of recovery of the homeostatic equilibrium is still very probable, whose biomarker is defined as b type and it is an index of low risk of pathology, (3) to the chronic inflammation, in which the recovery of the homeostatic equilibrium is physiologically improbable and the risk of degeneration or progression in different chronic-degenerative diseases is very high (the typical biomarker of this situation is defined as c type, and it is a index of a high-risk of pathology and of pathological progression)
These biomarkers could lead to advantages in the organization of the health system for improvement in the health quality, related to early diagnosis and personalized therapeutic treatments. They are, in fact, suitable for the prevention of healthy subjects as they allow the selection of individuals with a low or a high risk of pathology. An accurate evaluation of the clinical preventive procedure over these people is justified. Therefore, thanks to these indices, these procedures can be avoided in case they are unjustified. They are, also, prognostic for the stratification of the patients in clinical/therapeutic subgroups and for the development of the personalized medicine, allowing the quantification of the risk/benefit for the specific treatment that will surely lead to positive transformations in different clinical strategies.
3.3 New Biomarkers for the Treatment of Tumors
In oncology, the urgency for new suitable biomarkers is relevant: in spite of significant improvements in the clinical results of last decades, specific biomarkers that could be prognostic for the diagnostic and therapeutic monitoring and for the definition of personalized therapies are lacking. In fact, biomarkers are required in order to select the patients that, in the initial phase of the disease, could benefit from adjuvant therapies and to better define the clinical/therapeutic subgroups in advanced pathological phases.
The principal prognostic factor that is index of survival or recurrence after a surgery of a localized disease is, actually, the stage of the tumor [3, 4]. Adjuvant therapy is an antiblastic precautionary treatment, performed after a radical surgery (macro/microscopically), in order to eradicate any micrometastasis or cell in transition. At any rate, while stage I is usually cured with the surgery alone, the adjuvant chemotherapy is actually recommended for tumors at stage III and at stage II with a relatively high risk. However, about 75 % of patients in stages I–III could be treated with surgery alone. In stage III, 40–50 % of the individuals are cured in this way, while about 35 % of patients recur, in spite of receiving adjuvant chemotherapy [5]. Therefore, in clinical procedures performed on stage III, the majority of patients selected for adjuvant chemotherapy are cured, despite the fact that the majority of them do not require an adjuvant therapy or could not benefit from this treatment. The role of adjuvant chemotherapy is even more difficult to be defined in stage II disease: 60–70 % of these patients are treated with the surgery alone, and relapse is verified in 15–20 %, despite receiving adjuvant chemotherapy [6]. The QUASAR study randomized 3,239 colorectal cancer patients with a low risk for disease recurrence, to either observation or 5-fluorouracil/folinic acid (5-FU/FA); 92 % of these patients had stage II colorectal cancer. The 5-year beneficial effect of 5-FU/AF was only 3.6 %, indicating that 96 % of patients had received useless chemotherapy [7]. A surly valid contribution would be improvement in the quantification of the risk/benefit for a better treatment selection.
The aforementioned biomarkers, in the presentation of the intention of this chapter, could contribute to this improvement. More specifically (Fig. 3.2), a type biomarkers, which are homeostasis indices, are suitable for the identification of the subject that could benefit from the surgery alone; the b type biomarkers, indices of a transient inflammatory state and then of the possibility to restore their homeostasis, are suitable for the selection of the subjects that could benefit from adjuvant therapies in combination with surgery; and on the other hand, c type biomarkers, indices of a chronic inflammatory state in which the restoration of the homeostatic equilibrium is improbable, are suitable for the selection of subjects for which the adjuvant therapies represent an increase in the risk of worsening rather than a benefit. For these same characteristics, a, b, c type biomarkers are also suitable for the risk/benefit monitoring in the selection of a better treatment for the subjects in an advanced stage, for the stratification in clinical/therapeutic subgroup (Fig. 3.3).
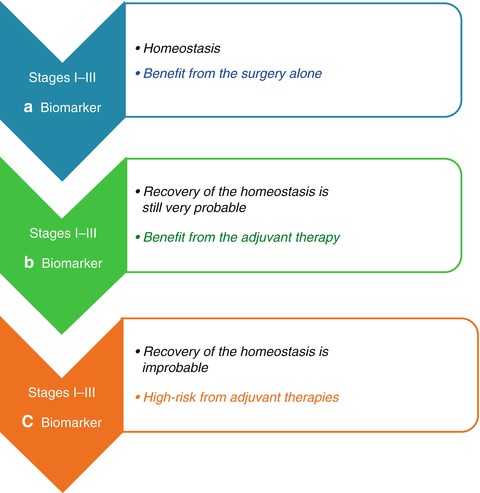
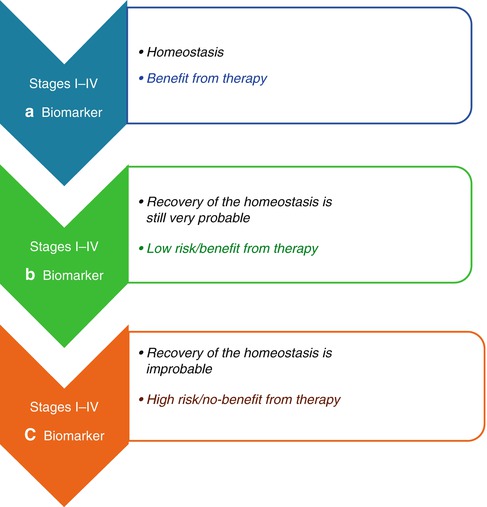

Fig. 3.2
Quantification of the risk/benefit for adjuvant chemotherapy. Adjuvant therapy is an antiblastic precautionary treatment, performed after a radical surgery (macro/microscopically), in order to eradicate any micrometastasis or cell in transition. At any rate, while the stage I is usually cured with the surgery alone, the adjuvant chemotherapy is actually recommended for tumors at stage III and at stage II with a related high risk. The role of adjuvant chemotherapy is even more difficult to be defined and a valid contribution would be the improvement in the quantification of the risk/benefit for a better treatment selection. The upper defined biomarkers could contribute to this improvement. More specifically the a type biomarkers, which are homeostasis indices, are suitable for the identification of the subject that could benefit from the surgery alone; the b type biomarkers, indices of a transient inflammatory state and then of the possibility to restore their homeostasis, are suitable for the selection of the subjects that could benefit from the adjuvant therapies in combination with surgery; on the other hand, c type biomarkers, indices of a chronic inflammatory state in which the restore of the homeostatic equilibrium is improbable, are suitable for the selection of the subjects for which the adjuvant therapies represent an increase of the risk of worsening rather than a benefit

Fig. 3.3
Quantification of the risk/benefit for a better treatment selection. (a–c) Type biomarkers are also suitable for the risk/benefit monitoring in the selection of a better treatment for subjects in an advanced stage, for the stratification in clinical/therapeutic subgroup
3.4 Guidelines for the Identifications of “Suitable” Biomarkers: A Healthy Longevity Is Linked to an Healthy Function of the Immune System. The Pathology Is Generated by Alterations of This System
As mentioned above, since aging of the population is a basilar cause of the increase in degenerative diseases such as tumor, in order to select “suitable” biomarkers, it is necessary to discover which substances bring to: (1) a healthy longevity, for the definition of prognostic biomarkers of a and b types, respectively related with a no-risk and a low-risk of pathology; (2) the degeneration in the pathology, to discover the prognostic biomarkers of the c type, indices of a high-risk of pathology.
For the identification of a correct research guideline in the comprehension of the relationship between the aging and a healthy longevity or with its related dysfunctions, it is important to consider that a healthy longevity is related with a healthy function of the immune system [8, 9] and that the pathology is generated by alterations of this system. In this contest, it is inevitable to consider that men and women not only follow different pathways for the regulation of the immune response homeostasis, but these pathways, specific for each gender, also suffer alterations during aging and also differently predispose the two genders to disease and to treatments [8].
Therefore, for the identification of prognostic suitable biomarkers for the development of concrete preventions and treatment strategies for men and women, it is necessary to separately perform the study in the two genders, examining significant parameters for (1) a healthy longevity and (2) a pathology degenerations.
3.5 The Importance of the Evaluation of Both Genders as Independent Groups
This necessity is based on clinical and experimental data showing that, when the immune system is involved, men and women could not be assessed in only one group, because the results wouldn’t be real, since in the immune response, there is a natural gender dimorphism [10–13].
During the reproductive ages, women have, for example, a more vigorous cellular and humoral immune response compared with men, and they also have more possibilities to reject transplants and tumors [14–19]. In addition, it has been proved that the immunosenescence affects both men and women, but it afflicts them differently. Men of every age and postmenopausal women present a less efficient T-cell response compared to premenopausal women [20]. Moreover, decrease of androgens in men during aging could contribute to their immunosenescence, but the loss of T-cell function, compared with women, is significantly less dramatic [21–23].
Multiple estrogen forms have been reported: estrone, estradiol, and estriol are the most common circulating forms. Estradiol binds both estrogen receptor-(ER)α and ERβ with high and equal affinities, while estrone preferentially binds ERα at a five times stronger affinity than ERβ [24]. Both pathways are involved in mediating estrogen effects, but ERα and ERβ present different functions inside the immune cells [25]. In premenopausal women, the estradiol derived from ovaries is the most common circulating estrogen, while estrone is the most common circulating form in postmenopausal women and in men. In men, testosterone is the primary substrate for estrogen production by the peripheral aromatization of androgens precursors, but it presents a modest difference related with age. However, the majority of studies have not revealed a significant correlation between age and the total level of estradiol in men [23].
Furthermore, it has been demonstrated that female and male hormones affect the immune system in opposite ways [26, 27]. For example, the Th1 and Th2 types of immune responses are respectively influenced, in prevalence, by androgens and/or estrogens: androgens favor the development of a Th1-type response and the activation of CD8+ cells [28], while estrogens seem to direct the immune system to a Th2 dominance, where B-lymphocytes are activated and antibodies are produced [27]. Pregnancy, a state in which estrogen level is high, is characterized, in fact, by a prevalence of Th2: when this condition is not preserved, there is an increased risk of abortion [29, 30]. Research has shown that gender, male or female, is associated with relevant incidence and prevalence of different types of age-related diseases and is an important variable in the genetics of longevity [31–33], indicating an important observation: men and women follow different strategies to reach longevity.
3.6 Men and Women Follow Different Strategies to Regulate the Homeostasis of the Immune System
3.6.1 Variations of Pro- and Anti-inflammatory Cytokine Levels Regulate the Immune Response and Could Influence the Healthy State
Research on cytokines is a valid tool for the study of the immune system as they are crucial for regulations of its correct function, being substances that regulate the transfer of information between cells thanks to the activation of membrane receptors.
Moreover, these results [33] confirm that during aging, there is a remodeling of the network level profile of cytokines produced by T helper cells (Th) in our physiological system, which is essential in the correct regulation of the immune system. Moreover, variations of the relationship between the levels of pro- and anti-inflammatory cytokines regulate the immune response which could influence the longevity and the healthy state during aging.
For these reasons, the analysis of the network level profile of the cytokines is a useful tool to define the prognostic biomarkers for aging [8, 9, 33–35]. In this area, results of recent studies [35–37] are relevant which indicate, for the first time, that the immune response is regulated by cytokines that differ between men and women, attributable to these dissimilarities the different trend, between male and female genders, of (1) the immune response, (2) the susceptibility to diseases, and (3) the therapeutic response, opening a new area for the translational research at this level. This study has shown that the gender dimorphism in the Th cytokine pathways is normally present in the regulation of the immune response homeostasis of the healthy state: the antigen-presenting cell (APC) regulates the differentiation and the homeostasis of different Th cell types in rest state and during activation of the immune system of both sexes, but this effect is exerted through Th cytokine pathways specific for each gender (gender-specific) but also through pathways that are common for both (gender-common).
These results [35–37] show, in fact, that the IFN-γ cytokine regulates the male immune system, while IL-6 cytokine regulates the female immune system. The correct functions of the IFN-γ in men and IL-6 in women are biomarkers for the ascertainment of a healthy state of the immune system and then of a healthy longevity. It has been shown, in fact, that an altered functioning of these pathways is a prognostic biomarker for the passage from a healthy condition to the genesis of an adenoma and the progression to a colorectal tumor [35]. On the other side, correct functioning of the IL-10 cytokine regulates the return of the immune system to the rest state, after the response, both in men and women, and, for this reason, it is a prognostic for a healthy longevity in the both sexes but only if IFN-γ in men and IL-6 in women are correctly functioning. If this condition is not respected, IL-10 is a biomarker for the progression of the tumor [30] both in men and women. These results have also revealed that [35] (i) the immune cell production of IL-6 in women and IFN-γ in men decrease during aging; (ii) the age could be a significant and independent factor for the pathways of IFN-γ and IL-10 in men (iii) and for the pathways of IFN-γ and the soluble molecule of the IL-6 receptor (sIL-6R, that modulate the action of IL-6) in women [38].
3.6.2 “Double Prognostic Biomarkers”: Appropriate Variations Between Pro- and Anti-Inflammatory Cytokines Assure the Success of the Immune Response but Following Different Gender Pathways
It has been shown that variations between pro- and anti-inflammatory cytokines could influence the success of the immune response [33–35]. However, the most relevant discovery for the definition of these suitable prognostic biomarkers is the identification of “double prognostic biomarkers”: they are constituted by couple of pro- and anti- inflammatory cytokines that differ between men and women and assure the success of the immune response varying in appropriate correlation with each other [35, 36] and following different pathways in each gender.
These results [35, 36] have been shown that the early evolution of the immune response is influenced by the positive correlation between the production of IFN-γ―IL-10 and IL-6―IL-4 in men and the negative one between IL-6―IL-10 in women. The evolution of the late response is also influenced by the positive correlation between the production of IFN-γ―IL-4 in men and IL-6―IFN-γ in women. More specifically, the “double prognostic biomarkers” for men are related by direct proportionality (they both increase or decrease together with the same trend, both positive or negative) between the IFN-γ―IL-10, IL-6―IL-4, and IFN-γ―IL-4 cytokine levels, while they are interconnected in women by an inverse proportionality (an increase of the first correspond to a decrease of the second and vice versa) between the IL-6―IL-10 cytokine levels and with a direct proportionality between IL-6―IFN-γ cytokines (Fig. 3.4). These variations between the couple of inflammatory and anti-inflammatory cytokines, specific for each gender, are to be considered “double biomarkers” gender-specific, in the valuation of relationships (1) between aging and a healthy longevity, to define the a and b types of indices, respectively of no and of low risk of pathology, for the healthy population prevention; of no and of low therapeutic risk for a better treatment selection of tumor patients; (2) between aging and pathology, to define the indices of c type with a high- risk of pathology for the healthy population prevention and a high therapeutic risk, for tumor patient treatments.
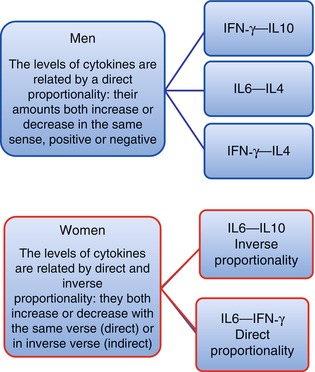

Fig. 3.4
The variations between pro- and anti-inflammatory cytokines influence the success of the immune response. The most relevant discovery is the identification of “double prognostic biomarkers,” which are constituted by couple of pro- and anti- inflammatory cytokines that differ between men and women and assure the success of the immune response varying in appropriate relationship with each other and following different pathways in each gender. These results have shown that the “double prognostic biomarkers” for men are related by direct proportionality (they both increase or decrease together with the same trend, both positive or negative) between the IFN-γ―IL-10, IL-6―IL-4, and IFN-γ―IL-4 cytokine levels, while they are interconnected in women by an inverse proportionality (an increase of the first correspond to a decrease of the second and vice versa) between the IL-6―IL-10 cytokine levels and with a direct proportionality between the IL-6 ―IFN-γ cytokines. These variations between the couple of inflammatory and anti-inflammatory cytokines, specific for each gender, are to be considered “double biomarkers” gender-specific, in the evaluation of relationships (1) between aging and a healthy longevity, to define the a and b types of indices, respectively of no and of low risk of pathology, for the healthy population prevention; of no and of low therapeutic risk for a better treatment selection of tumor patients; (2) between aging and pathology, to define the indices of c type with an high risk of pathology for the healthy population prevention; and a high therapeutic risk, for tumor patients treatments
The relevance of the gender-specific differences in the regulation of the immune response is underlined by the evidence that homeostasis is preserved and there are no differences between men and women in the outcome of this response when the pathways of the gender-specific cytokines (IFN-γ e IL-6) still normally work [35, 36]. When alterations occur in the pathways of the gender-specific cytokines, the consequences for men and women are different, in terms of disease development. This event is related to the impairment of the immune system homeostasis, because the alterations of gender-specific cytokine pathways cause a pathologic polarization of specific T-cell types, different for each gender. The reason of this fact is related to the different effects on the generation of Th cell subtypes during the immune response generated by IFN-γ and IL-6 cytokines, present in the cellular environment. IFN-γ sustains the development of the Th1 type [39], while IL-6 supports the Th2-type differentiation and the activation of B-lymphocytes with the related production of antibodies. In addition, research in this area showed that it is not a single cytokine to determine a particular response but the interactions between different individual cytokines, organized in a network. These results [35, 36] indicate that the different predisposition to gender-specific diseases and the related disease progression are related to different cellular gender-specific polarization of the Treg, Th17, and Th9 cells, determined by interactions between TGFβ, IL-6, IFN-γ, IL-10, and IL-4 cytokines that vary between men and women [36]. These results are confirmed by the evidences of other researches that indicate the existence of mutual relationships in the Treg, Th17, and Th9 cells, because (i) TGFβ triggers the expression of Foxp3 transcription factor in naïve T cells, generating Treg cells, but (ii) IL-6 inhibits the TGFβ-driven expression of Foxp3, and TGFβ together with IL-6 induces ROR-gt transcription factor, triggering the developmental program of Th17 cells [36, 40], while (iii) IL-4 also inhibits TGFβ induction of Foxp3 expression, but TGFβ together with IL-4 induces Th9 cells’ differentiation that produce IL-9 cytokine. The co-expression of IL-9 and IL-17 has been identified as a new function of Th17 in the mediation of tissue destruction and of the neurodegeneration [41, 42]. The female greater susceptibility to autoimmune diseases, as multiple sclerosis, could be attributed to the dominant influence of IL-6, in the inhibition of the regulatory cells that play a fundamental role in these diseases [40, 43–45]; the greater male susceptibility to, for example, the primary form of progressive multiple sclerosis (MS) [46] could be the result of the prevalent influence of IFN-γ in the control of Th9 cells that predispose to neurodegeneration. The IL-9 receptor complex is constitutively expressed on the astrocytes, and IL-9 induces the expression on these cells of CCL-20 but not the other chemokines [47], determining the migration of Th17 cells in the CNS where they promote neurodegeneration. Treg, Th9, and Th17 cells have shown to be important in the autoimmune diseases, as rheumatoid arthritis [48] and MS [49].
3.6.3 The Efficiency of the Treatment Is Related to a Reestablishment of IL-6 Pathways in Women, and IFN-γ Pathways in Men
Results in the research on MS [36] confirm these data, showing that alterations of the IL-6 pathways are involved in the loss of homeostasis of the immune system which is evident in a disequilibrium of Treg cells and in an increase of the neurological deficit in both gender, underlining the autoimmune etiology of MS. Further support of the existence of gender-specific pathways is provided by this study, observing that the benefit of the treatment with IFN-β (in the reestablishment of the immune system homeostasis and in the inhibition of the neurological deficit) is related to the reestablishment of IL-6 pathways in women and IFN-γ pathways in men. Moreover, the serum levels of the soluble molecule sCD30 and of the TGFβ cytokine could be used in both genders as “double biomarkers,” for the assessment of the therapeutic success in terms of reestablishment of the immune homeostasis of the Th cells and the absence of the progression of neurological deficit.
Overall, these results shed light on the necessity of (a) suitable gender-specific biomarkers and (b) gender-specific drugs, whose activity could consider the different regulation system of the immune response between the two genders, assuring the same therapeutic results: return to the physiologic homeostasis thanks to the passage from an activation pathological state to a rest one.
On these bases, it is possible to affirm that the definition of suitable gender-specific biomarkers could lead to new strategies of preventions and to personalized treatments [37].
3.7 The Valuation of the Thioredoxin and CD30 Systems for the Prognostic, Diagnostic, and Therapeutic Stratification of Patients
It has to be considered that specific mechanisms responsible for different disease susceptibility between men and women have still to be clarified. However, research suggests that response could be found in different capacities of male and female cell to defend themselves from the oxidative stress [50, 51]. The cells of men and women differ in terms of production of reactive species of oxygen (ROS) and susceptibility to the oxidative stress [51]. This concept constitutes a new enthusiastic research area. Oxygen metabolism could lead to the production of ROS in every type of cell, including cells in the immune system, that present antioxidant compounds and enzymes (as glutathione and thioredoxin reductase) [52, 53] and able to neutralize ROS and preserve the cellular oxidative equilibrium. However, the activity of ROS seems to be regulated in different ways between men and women and could be directly influenced by sexual hormones [51].
In vivo studies have also revealed the incapacity in male, but not in female, in the maintenance of reduced intracellular redox condition, essential for normal cellular functions [50], and this circumstance explains, at least in part, the differences between the two genders of the Th gender-specific cytokines pathways in the regulation of the immune system homeostasis. IFN-γ pathways, specific for male, is, in fact, a direct stimulator of the thioredoxin (Trx) and thioredoxin reductase (RTrx) gene expression in human T cells [53, 54], and there is a positive feedback between IFN-γ and the genetic expression of Trx/RTrx in the intracellular oxidative reduced regulation that is essential for the immune response. For these reasons, it is correct to suppose that the immune response through the IFN-γ pathway in men is indispensable for the activation of the Trx1/RTrx1 system and for the decrease of the intracellular oxidation levels, in order to preserve the oxidative cellular equilibrium. Indeed male cells, as previously pointed, are not able to maintain the intracellular oxidative reduced condition.
Therefore, these results indicate that for the identification of male and female gender-specific targets and biomarkers in an easy and early way in association with the onset of the disease, it is also required to valuate, in the peripheral blood, the factors that regulate the redox system and that intervene in multiple cellular process, as proliferation, cellular cycle, and death or survival signal pathways [55–58]. Indeed for the neoplasia, these processes are really relevant because they both interfere in the regulation of the host response to the tumor and the tumor to the host [59–63], and for these characteristics, they are potential targets/biomarkers with an ample predictive capacity in the clinical, diagnostic, and therapeutic stratification of oncologic patients. The use of these targets to identify new drugs to reestablish the physiologic homeostasis of the apoptotic process between the immune cell and the tumoral tissue is also fundamental for the definition of therapies specific for the regression of the neoplasm.
The immune-modulatory role of the oxidative stress is a support to the importance of the redox system in this contest. The oxidative stress is defined as cellular toxicity caused by oxygen derived free radicals and it determinates the loss of homeostasis in the redox cellular system and the functional body, as it makes the extra/intra-cellular environment balance impossible, which is vital for the normal functional body response [61]. Indeed, in homeostasis, the cell is able (1) to balance the activity of the oxidant/antioxidant factors, (2) to maintain reduced intracellular redox environment [56, 57], and then (3) to assure the normal functionality. In case of alterations in the redox system, the cell is no more able (1) to balance the relationship between oxidant/antioxidant factors, (2) to adapt the extra/intracellular environment and, for this reason, (3) the intracellular redox system is not reduced and the organism could not respond to the environment necessity, or it perform these activities in an inappropriate way [64]. In addition, it has to be underlined that for the biggest part of tumor agents, including the radiant therapies, the cytotoxicity for the tumor regression is concretized trough the induction of oxidative stress that originates from cellular injury caused by intermediary oxidant factors of the redox system [65]. A support of this hypothesis is furnished by the evidence that in tumors, including colorectal cancer, alterations in the physiological pathways of redox system regulations have been identified, and the results show that the functional inactivation of the immune cells produced by free radicals represents a significant immunosuppressive mechanism of the cellular response for the defense against the tumor [66–70].
Essential redox factors for the immune response are the ones appertaining to the thioredoxin system (Trx) which is a physiologic and fundamental regulator of the redox-mediated cellular reactions. Trx1 is a protein-containing selenocysteine and it catalyzes the NADPH-dependent reduction of the thioredoxin reductase (RTrx1) with other numerous oxidized cellular proteins [71–73]. After an oxidative stress, Trx1 produces different cellular signals that activate the specific transcription factors regulating the nuclear decode of genes involved in the production of substances able to defend the cell against the oxidative stress induced by free radicals [74, 75].
On these bases, it is clinically relevant that CD30 (a membrane receptor of the immune cells as T and B cells, monocytes, dendritic cells, NK, eosinophils, and granulocytes) is the specific Trx1 immune receptors [76]. The potential of Trx1 and CD30 as single targets and biomarkers has yet been explained in the literature and in tumors, but the innovative hypothesis is the combined use of Trx1 and sCD30 (the soluble molecule of CD30 receptor) as a double target/biomarker (Trx1-CD30) [77]. The rational of this new direction is that the double target/biomarkers together with immunological and genetic-related parameter concretize (1) the availability of a composition of “suitable prognostic biomarkers” that are gender-specific and gender-common, for the prevention programs on healthy population and for the stratification of patients in clinical/therapeutic subgroups or to obtain personalized treatment; (2) it could also be the guideline for pharmacological interventions on the redox, immunological, and neurological systems, in chronic-degenerative states as tumor [77] and aging [37].
3.7.1 Trx1/RTx1 System
The redox control of the cellular physiology is one of the most relevant regulatory mechanisms in every living organism. Mammal cells contain two Trx-systems. The first is the Trx1/RTx1 that is normally localized in the cytoplasm but, in stress condition, could migrate into the nucleus (inducing the genetic codification) or it could be secreted in the extracellular environment [78] and, in this way, it attends the immune system network. The second system (Trx2/RTrx2) is localized in mitochondria and in the cytoplasmic reticulum, and it regulates cellular apoptosis [79]. There are two other system: the first is called TRXs testis/sperm-specific and is localized on the spermatids (Sptrx-1, Sptrx-2, and Sptrx3), and the second is the Txl-2 localized in lungs and in other ciliate tissues [80].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree