History and physical examination
Laboratory tests: CBC, CMP, LDH, urine analysis, PSA in men
Computerized tomographic (CT) scans of the chest, abdomen, pelvis
Positron emission tomography (PET) scan in selected patients (squamous carcinoma in cervical/inguinal nodes and others with a single site of involvement)
Mammography in women
Pathology evaluation: including screening immunohistochemical stains of the biopsy on carcinomas (CK7, CK20, TTF-1, CDX-2)
Additional clinical and pathologic evaluation based on details from history, physical examination, laboratory testing, medical imaging, and specialized pathology (Table 11.4)
If an anatomical primary site found the patients do not have CUP
Molecular cancer classifier assay on very small biopsy/aspiration/cytology specimens or when immunohistochemical stains not diagnostic of a single cancer type or tissue of origin
Table 11.2
Additional evaluation determined by findings from initial diagnostic evaluation
Results of initial diagnostic evaluation | Additional evaluation | |
|---|---|---|
Clinical | IHC staining/other testing | |
Features of lung carcinoma (hilar/mediastinal adenopathy; biopsy positive for CK7 and TTF-1) | Bronchoscopy | EGFR mutation, FISH for ALK, ROS1 rearrangement |
Features of colorectal carcinoma (liver/peritoneal metastasis; biopsy positive for CK20 and CDX2) | Colonoscopy | KRAS mutation test |
Features of breast cancer in women (axillary nodes, lung, bone, liver metastasis; biopsy positive for CK7) | Breast MRI | ER, GCDFP-15, mammoglobin, GATA3, FISH for HER2 |
Features of ovarian cancer in women (pelvic/peritoneal metastases; CK7+) | Pelvic/intravaginal ultrasound | WT-1, PAX8, ER |
Mediastinal/retroperitoneal mass in young adults | Testicular ultrasound, serum AFP, HCG, LDH | PLAP, OCT4; FISH for i(12)p |
Poorly differentiated carcinoma, with or without clear cell features | Serum AFP if Hepar1+; octreotide scan if neuroendocrine stains+ | Chromogranin, synaptophysin, RCC, PAX8, Hepar1, MelanA, HMB-45 |
Liver metastasis predominant (CK7-,CK20-) | Serum AFP | Hepar1 |
Any histology without a single cancer site or tissue of origin predicted by IHC | Molecular cancer classifier assay | |
Table 11.3
Immunohistochemical staining patterns of a biopsy characteristic of a single cancer or tissue of origin
Prostate | CK7−, CK20−, PSA+ |
Lung adenocarcinoma and large cell | CK7+, CK20+ TTF-1+, Napsin A+ |
Lung neuroendocrine (small cell/large cell) | Chromogranin+, synaptophysin+, CD56+, TTF-1+ |
Thyroid carcinoma (papillary/follicular) | Thyroglobin+, TTF-1+ |
Melanoma | MelanA+, HMB45+, S100+ |
Adrenal carcinoma | Alpha-inhibin+, Melan-A+(A103) |
Renal cell carcinoma | RCC+, PAX8+ |
Germ cell carcinoma | PLAP+, OCT4+ |
Ovary carcinoma | CK7+, WT-1+, PAX8+, ER+ |
Hepatocellular carcinoma | Hepar-1+, CD10+, CD13+ |
Breast carcinoma | ER+, GCDFP-15+, mammaglobin+, GATA-3+ |
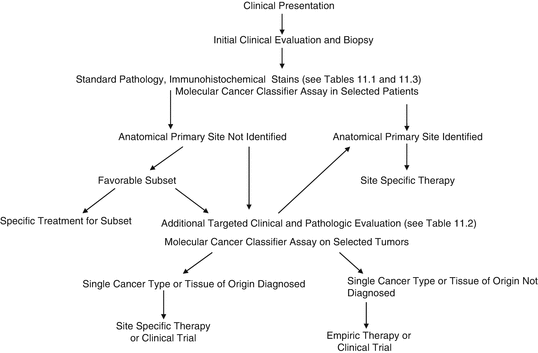
Fig. 11.1
Evaluation of a possible CUP patient
Prior to 2008, the precise cancer type for the majority of CUP patients could not be determined, but was presumed in a minority of patients within several subsets which were recognized over many years as favorable subsets. (For example, women with metastatic adenocarcinoma involving axillary lymph nodes were presumed to have breast cancer; patients with metastatic squamous carcinoma involving upper or mid-cervical lymph nodes were presumed to have a head/neck primary site; etc.) However, the use of IHC stains and molecular cancer classifier assays performed on the biopsy specimen now results in a diagnosis of the tissue of origin/type of cancer for most CUP cancers.
11.2.1 Immunohistochemical Staining
Immunohistochemistry has improved over the past several years with the discovery of increasingly specific cellular proteins. In addition, panels of IHC stains have been useful (Table 11.3) in diagnosing a number of cancers based on relatively specific tumor staining profiles [3, 4]. False-negative and false-positive stains are relatively common, and the characteristic staining patterns shown in Table 11.3 are not always observed in those cancer types; positive and negative staining may overlap in different tumors. The prediction of a single-specific cancer type or tissue of origin by IHC staining is much more reliable than several years ago, but such a prediction is still not possible in about 60 % of CUP cancers [3, 6, 17].
The role of IHC staining in the management of CUP has developed over many years, and certain results are now accepted and used to make treatment decisions. For example, IHC stains are used to evaluate poorly differentiated neoplasms, and when a lineage is established (e.g., lymphoma, germ cell tumor, neuroendocrine carcinoma, thyroid carcinoma, melanoma), appropriate treatments are instituted. Although the clinical validity of this practice has been supported by small, retrospective trials, the use of IHC staining results to direct therapy has never been evaluated or validated in prospective studies. Paradoxically, several common epithelial cancers (e.g., lung, colorectal, breast, kidney) now have rather specific diagnostic IHC staining patterns (Table 11.3), yet these IHC results have not been widely used to direct treatment for these CUP subsets. We call attention to these inconsistencies not to minimize the contribution of IHC but to provide a backdrop for the current controversies regarding the studies required to establish molecular profiling results as integral in directing CUP treatment (see below).
11.2.2 Molecular Cancer Classifier Assays
Molecular cancer classifier assays, based on tissue-specific patterns of gene expression, have been developed to diagnose the specific tissue of origin by testing the biopsy from a metastatic site. These molecular assays complement IHC staining [16–21] and are particularly useful when IHC stains are not conclusive [3, 14, 17–19, 21, 31]. Several molecular cancer classifier assays have been studied in CUP [3, 5, 11–15, 22–26, 31], but most information has been reported from three assays [92-gene RT-PCR assay, CancerTYPE ID [7]; microarray for microRNAs, Cancer Origin Test [9]; microarray for mRNA, Tissue of Origin Test [8]].
There have been no direct comparisons of these assays, but their ability to diagnose the specific tissue of origin in CUP is now well documented [3, 5–21, 31]. Since the occult anatomical primary sites could not serve as a gold standard of reference, the accuracy and diagnostic utility of molecular assays have been evaluated by indirect methods (clinicopathologic comparisons). In one study of 171 CUP patients, the accuracy of the 92-gene RT-PCR assay was evaluated by three separate methods [17]: (1) direct comparison to latent primary sites found months to years later, (2) comparison to single IHC staining diagnoses, and (3) obtaining additional confirmatory/supportive IHC staining based on the molecular assay diagnosis. An accuracy rate of about 80 % was determined using each of these 3 evaluative methods [17].
Molecular cancer classifier assays are therefore not 100 % accurate, and the limitations of their ability to make precise diagnoses are now well recognized [17]. All data, including clinical information (age, gender, imaging studies, laboratory values, sites of metastasis), tumor histology, and IHC staining results (initially and if necessary as confirmatory/supportive testing after a molecular assay diagnosis), should be considered in concert with the molecular assay result to determine the specific cancer type or tissue of origin. When all available data are considered, the tissue of origin can be identified or highly suspected in about 95 % of patients with CUP.
11.2.3 Comparisons of IHC Staining and Molecular Cancer Classifier Assays
Two relatively large studies have compared molecular cancer classifier assays and IHC staining in their respective abilities to diagnose a cancer type or tissue of origin [19, 21]. Both studies were performed on biopsies from patients with metastatic cancer of known origin; pathologists were given biopsy specimens and informed of the site of the biopsy and the gender of the patient. The specimens were large, and the pathologists could perform as many IHC stains as felt necessary for an optimal diagnosis. They were asked to predict a single cancer or primary site even if the IHC staining results were not completely characteristic or diagnostic. A molecular cancer classifier assay, the 92 gene RT-PCR (CancerTYPE ID) in one study [19] and the microarray mRNA assay (tissue of origin) in the second study [21], was performed on each biopsy specimen.
The results of these two studies are illustrated in Table 11.4. The primary sites of most tumors were correctly identified by both IHC staining and the molecular assays. In the study using the 92 gene RT-PCR assay, a median of 7.9 IHC stains/tumor were used, and 84 of 122 tumors (69 %) were correctly diagnosed compared to 96 of 122 tumors (79 %) for the molecular assay [19]. In the study using the microarray mRNA assay, the median number of IHC stains was 8.3 stains/tumor; IHC staining correctly diagnosed 83 % of 157 tumors versus 89 % for the molecular assay [21]. The molecular assay was more accurate in diagnosing poorly differentiated carcinomas (91 % vs. 71 %). In both studies, the diagnostic accuracy of IHC decreased (to about 70 %) once greater than 8 stains were performed.
Table 11.4
Comparison of the accuracy of IHC versus molecular cancer classifier assays in identification of metastatic tumors of known primary site
Known Primary site | Handorf et al. [21] | Weiss et al. [19] | ||||
|---|---|---|---|---|---|---|
Number of specimens | Accuracy (%) | Number of specimens | Accuracy (%) | |||
aIHC | MCCA | aIHC | MCCA | |||
All | 157 | 83 | 89 | 122 | 69 | 79 |
Poorly differentiated histology | 33 | 71 | 91 | —— | aNR | NR |
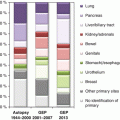
Lung | 6 | 100 | 83 | 24 | 67 | 75 |
Colon | 25 | 92 | 100 | 17 | 94 | 94 |
Breast | 25 | 84 | 100 | 11 | 55 | 73 |
Kidney | 14 | 100 | 86 | 13 | 77 | 77 |
Bladder | 10 | 43 | 60 | 11 | 45 | 82 |
Ovary | 8 | 75 | 88 | 5 | 100 | 100 |
Stomach/esophagus | 7 | 29 | 29 | 5 | 60 | 60 |
Prostate | 5 | 56 | 100 | 4 | 50 | 100 |
Pancreaticobiliary | 3 | 73 | 60 | 4 | 75 | 50 |
In both of these comparative studies, the spectrum of tumor types included may have influenced the results in favor of IHC staining. The majority of cancers originated in the lung, breast, ovary, kidney, or colon, all sites with relatively specific IHC staining patterns. In contrast, several tumor types lacking specific IHC staining patterns but common in the CUP population (e.g., stomach, biliary tract, pancreas, gastrointestinal junction, urothelial carcinoma) were not well represented.
The use of IHC staining and molecular assays has also been reported in a few studies in patients with CUP [3]. In 5 reported studies, biopsies from a total of 117 patients were evaluated with IHC and molecular profiling [12, 14, 17, 32, 33]. In approximately 50 % of cases, IHC staining led to prediction of a single tissue of origin. In 78 % of these cases, molecular profiling yielded an identical prediction. However, molecular profiling gave a tissue of origin prediction in 96 % of all patients and was therefore useful in many of the tumors that were not characterized by IHC.
11.3 Treatment of Patients with CUP
11.3.1 Background
Patients who are classified within several recognized favorable CUP subsets (about 20 % of all CUP) are treated with specific regimens based upon their presumed tissues of origin (see Chap. 10). In general, these treatments are site specific, based on the probable site of tumor origin in these patient subsets. Examples include treatment of women with adenocarcinoma involving axillary lymph nodes as breast cancer, and treatment of patients with squamous carcinoma in cervical lymph nodes as head/neck cancer. Such treatments have resulted in improved survival in these patient subsets.
The majority of the remaining CUP patients (approximately 80 % of all CUP) have received empiric chemotherapy regimens; prognosis for most of these patients has remained poor. Empiric chemotherapy regimens (i.e., paclitaxel/carboplatin, gemcitabine/cisplatin, others) were developed because the specific cancer type or tissue of origin was not known and for most patients could not be determined. Empiric chemotherapy became the standard of care in the mid-1990s after clinical trials showed benefit at least for some patients [34]. At that time, CUP was considered by many as a single entity with a similar clinical biology, rather than a heterogeneous group comprising many types of cancer with varying behavior. Large trials (100 or more patients) of empiric chemotherapy for the unfavorable group of CUP patients have invariably resulted in median survivals of about 9 months and 2-year survivals of 10–20 % [34]. Those patients with benefit likely harbored more sensitive cancer types (such as breast or ovarian cancer), while treatment was ineffective for those with unresponsive cancers types (such as biliary tract, pancreas).
A few clinical trials several years [1, 2] ago incorporated a few targeted type drugs (first-generation drugs) alone (bevacizumab, erlotinib) or combined with chemotherapy (paclitaxel/carboplatin) but without any knowledge at that time of specific targets within the cancer cells. These endeavors, although producing some tumor responses, were essentially empirical approaches based on activity and benefit seen in a few known advanced solid tumors (non-small cell lung, colorectal).
Recent improvements in therapy have resulted in improved survival for patients with many types of known advanced cancers. These therapies have also become more individualized or specific for each particular cancer type. Furthermore, genetic alterations are now recognized in many cancers and provide the basis for effective precision or targeted therapies for selected patients. Therefore, the recognition or diagnosis of the specific cancer type or tissue of origin in each CUP patient may have critical therapeutic implications. The optimal treatment today for patients with advanced cancers requires the identification of the site of origin or specific type of cancer.
11.3.2 Site-Specific Therapies Based on Tissue of Origin in CUP
The ability to diagnose the tissue of origin in the majority of CUP patients has inaugurated an era of site-specific therapies rather than the continued use of empiric chemotherapy. Three critical questions needed to be addressed and resolved in order to confidently change the standard of care. First, can molecular cancer classifier assays accurately provide a specific tissue of origin diagnosis? Second, can molecular assays complement standard pathology? Finally, do patients have a better survival if they are treated with site-specific therapies based on a tissue of origin diagnosis versus empiric chemotherapy?
As previously reviewed in this chapter, the accuracy of molecular cancer classifier assays and their ability to complement standard pathology are firmly established. Molecular cancer classifier assays, when used with IHC and the clinical features, facilitate a specific/single tissue of origin diagnosis in 95 % of patients, and about 80 % of these diagnoses are accurate [1–3, 17]. The molecular assays complement IHC staining and frequently make a single diagnosis when IHC is inconclusive [3, 19–21, 25, 26, 31].
Conclusive demonstration of the superiority of site-specific versus empiric therapy in CUP patients is difficult for a number of reasons. There are at least 30 different types/subtypes of metastatic cancers within the CUP population. About half of these are relatively resistant to therapy (e.g., pancreas, biliary tract, liver, others) and can be expected to do poorly regardless of the treatment administered. Many of the CUP patients with more responsive cancers (e.g., breast, ovary, lung) would derive some benefit from empiric taxane/platinum regimens (although without the benefit of site-specific targeted agents and sequential useful therapies), while in only a small percentage (e.g., colorectal, renal) would site-specific and empiric CUP therapy diverge markedly. Given these considerations, a randomized trial comparing empiric chemotherapy versus assay-directed site-specific therapy, as advocated by some, would require a minimum of several hundred patients. Fewer patients would be required if enrollment was limited to potentially responsive subtypes, but enrolling these patients may pose an ethical dilemma for some physicians.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree