(1)
Institut Bergonié, Bordeaux, France
Abstract
The Notch pathway, as the Wnt–β-catenin pathway, is an important signalling pathway in vertebrate and invertebrate development. It is involved in embryogenesis and morphogenesis and plays a major role in the fate of stem cells to differentiation. The loss of function of some of its actors leads to hereditary malformations, and activating mutations and/or overexpression are found in malignant diseases.
Briefly, protein messengers called DSL (Delta, Serrate, Lag2, after the names of the ligands found, respectively, in mammals, Drosophila and Caenorhabditis elegans) recognise and activate membrane receptors called NOTCH, this activation consisting in receptor cleavage. The cleavage products can then migrate into the nucleus and activate the transcription of target genes through inhibition of transcriptional repressors. One of the important features in this pathway is the juxtacrine interaction of ligands and receptors, both ligands and receptors being transmembrane proteins: the presentation of a ligand to a neighbour cell induces the activation of the receptor cell.
8.1 DSL Ligands
Two families of DSL ligands can recognise the NOTCH receptors in mammals: DLL (delta-like ligands) and JAG (jagged), which correspond to the Serrate ligands of Drosophila. These ligands are type I transmembrane proteins (i.e., their N-terminal part is in the extracellular space).
DSL ligands have a short intracytoplasmic domain, a single transmembrane domain and a large characteristic extracellular domain, made of 6–14 tandem repeats of EGF-like domains, a DOS (delta and OSM-11-like) domain absent from DLL3 and DLL4 and a DSL domain. DLL3 and DLL4, devoid of a DOS domain, require a DLK (delta-like protein homologue) coligand, equipped with DOS and EGF-like domains but devoid of a DSL domain. The simultaneous presence of DOS and DSL domains appears necessary for the ligand–receptor interaction, the DOS domain being either directly brought by the ligand (for JAG1, JAG2 and DLL1) or by a coligand (for DLL3 and 4). The protein structure of ligands and coligands of the Notch pathway is presented on Fig. 8.1.
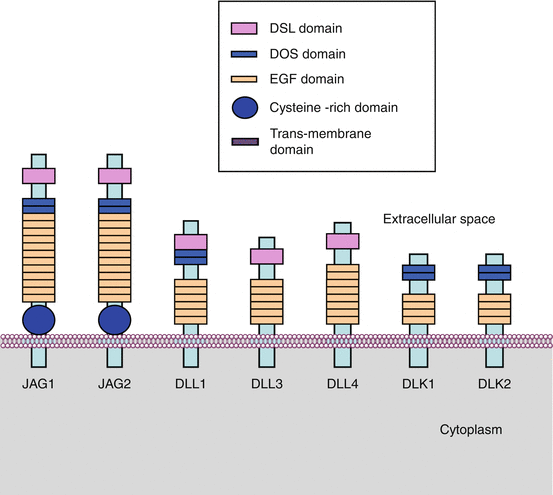

Fig. 8.1
DSL ligands. DSL ligands contain a series of domains that are essential for their function: the DOS and DSL recognition domains and 6–14 EGF-like domains. The JAG ligands contain in addition a cysteine-rich domain. DLL3 and DLL4 have no DOS domain and depend upon the presence of the coligands DLK1 and DLK2, which harbour a DOS domain but no DSL domain
8.2 NOTCH Receptors
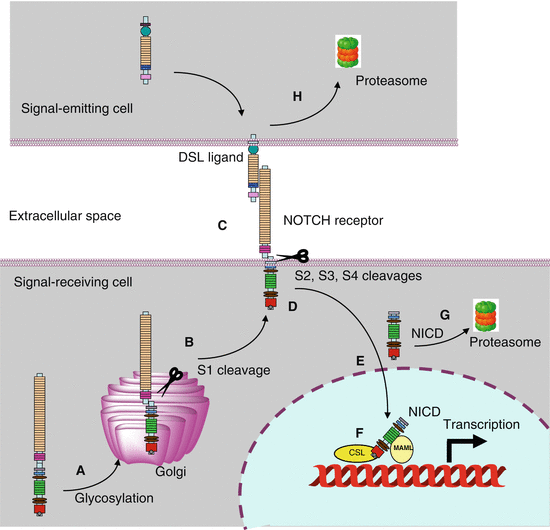
DSL ligands are recognised by NOTCH receptors; four NOTCH receptors exist in humans, which are also type I transmembrane proteins. Whereas the protein sequence in Drosophila is continuous and covers the extra- and intracellular parts, NOTCH receptors are heterodimeric in mammals, the monomers being generated in the Golgi apparatus by post-translational cleavage of a precursor containing the whole sequence, followed by non-covalent reassociation of the two monomers. The cleavage is realised by furin, a member of the subtilisin-like proprotein convertase family at a site called S1, and the resulting monomers are called NECD (notch extracellular domain) and NTMICD (notch transmembrane and intracellular domain).
In their extracellular part, the NOTCH receptors consist of 29–36 tandem repeats of EGF-like motifs, which contain O-glycosylation sites for fucose or glucose residues. The EGF-like motifs are followed by a NRR (negative regulatory region) sequence consisting of three tandem repeats of LNR (lin12 and notch repeats) motifs and a heterodimerisation (HD) domain where the S1 cleavage site is located. After the transmembrane domain, the intracellular part contains a RAM (RBPJK [recombination signal binding protein for immunoglobulin kappa J region]–association module) motif, followed by nuclear localisation sequences (NLS), ankyrin repeats (ANK) and a transactivation domain harbouring PEST (proline–glutamic acid–serine–threonine-rich) domains. Figure 8.2 presents the general structure of the NOTCH receptors.
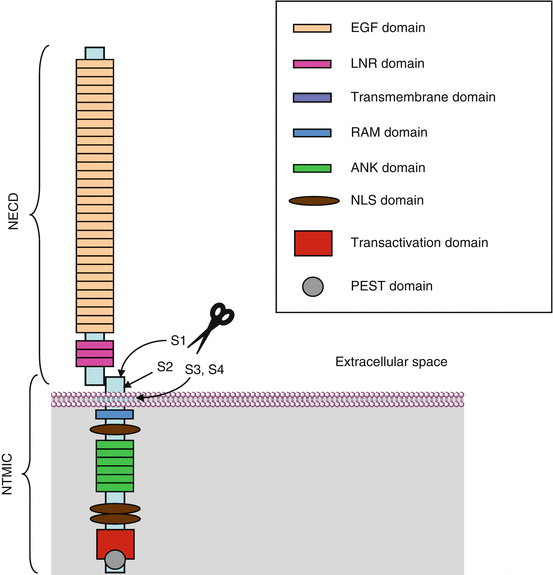

Fig. 8.2
NOTCH receptors. The four NOTCH heterodimeric receptors are built on the same model and associate an extracellular domain (NECD) and a transmembrane and intracellular domain (NTMICD), tied together via a non-covalent interaction at the level of a heterodimerisation domain HD; the two monomers result from the cleavage of a unique precursor protein, which is cleaved within the HD domain in the Golgi apparatus by furin. NECD contains 29–36 EGF-like domains and three LNR domains involved in the negative regulation of the Notch signalling pathway by protecting the S2 cleavage site of the HD domain. NTMICD contains a RAM domain, involved in the interaction with DNA-binding proteins, together with nuclear localisation sequences NLS, ankyrin repeats ANK involved in the recruitment of the transcriptional activator MAML and a transactivation domain containing PEST motifs
8.3 NOTCH Receptor Activation and Signal Transmission
Ligand–receptor binding occurs by homotypic interaction of the EGF-like domains and induces the proteolytic cleavage of the receptor by a metalloproteinase, ADAM10 (a disintegrin and metalloproteinase) or ADAM17 (also known as TACE, TNF-alpha converting enzyme), at the level of the site S2 located within the heterodimerisation domain, thus in the extracellular part of the receptor, 12 amino acid residues ahead of the transmembrane domain. This cleavage is possible thanks to a conformational change releasing the S2 site from the protection exerted by the LNR domains. The intermediate protein is called NEXT (notch extracellular truncation) and is the substrate of the proteolytic activity exerted by the γ-secretase complex, at two transmembrane sites called S3 and S4. After cleavage, the NOTCH intracellular domain (NICD) is able to migrate to the nucleus, thanks to the unmasking of the NLS. γ-secretase is a multimeric transmembrane complex harbouring presenilins 1 and 2 (genes PSEN1 and 2), nicastrin (gene NCSTN) and activation and stabilisation factors of these proteases.
In the nucleus, NICD interacts, thanks to its RAM domain, with a DNA-binding protein of the CSL (CBF [Core-binding factor-1] /su(H)/lag-1) family, which is RBPJK in humans. The ANK domain then recruits a coactivator called MAML (mastermind-like protein), which in turn recruits the MED8 (mediator of RNA polymerase II transcription, subunit 8) factor, allowing the transactivation of target genes. Figure 8.3 presents the steps of the post-translational maturation of the NOTCH receptors, interaction with DSL ligands, proteolysis, migration of the active part to the nucleus and interaction with activating and repressing transcriptional complexes.