Analyte
3 months previously
Current presentation
Reference range
Calcium
9.5
9.6
8.9–10.1 mg/dL
Phosphorus
1.9
2.0
2.5–4.5 mg/dL
Creatinine
0.5
0.7
0.6–1.1 mg/dL
Parathyroid hormone
103.5
81.2
15–65 pg/mL
25-hydroxyvitamin D
21
25.3
20–50 ng/mL
1,25-dihydroxyvitamin D
–
71
18–78 pg/mL
TSH
0.97
–
0.3–4.2 mIU/L
24-h urinary calcium
280
390
25–300 mg/24 h
24-h urinary phosphorus
–
1347 mg
<1100 mg/24 h
Review of her records indicated a highest calcium level (10.5 mg/dL) approximately 6 months prior to the current evaluation, when she had been taking up to ten tablets of calcium carbonate per day (1000 mg) for self-management of heartburn and reflux. This had been stopped due to the elevated calcium value, and she has not been maintained on any calcium supplementation since that time.
Assessment and Diagnosis
Primary hyperparathyroidism (PHPT) has been well recognized as a very common endocrine disorder with well-characterized diagnostic and prognostic features since the early 1970s when serum metabolic panels were introduced for routine screening that could detect hypercalcemia in otherwise asymptomatic patients. Over the past two decades, normocalcemic primary hyperparathyroidism (NPHPT) has been identified as a new subset of this disease in what Silverberg and Bilezikian referred to as a “forme fruste” of PHPT, identified primarily in patients referred for evaluation of low bone mass or less commonly nephrolithiasis [1].
NPHPT is defined by elevated PTH levels that occur concomitant with normal albumin-corrected serum calcium levels, after exclusion of secondary hyperparathyroidism [2] as an etiology. In contrast to PHPT patients in whom normal calcium levels may occur intermittently, patients with NPHPT are consistently normocalcemic on repeated evaluations. As discussed below, some patients may progress to overt hypercalcemia; such patients would then be considered to have progressed to PHPT [3].
NPHPT should be first suspected in patients having an elevated PTH level with consistently normal albumin-corrected serum calcium levels. However, several nuances to these diagnostic features are to be noted.
First, several sources suggest ensuring normal ionized calcium as well [4, 5]. Up to 10 % of patients with PHPT can have normal total serum calcium and elevated ionized calcium levels [6]. Obtaining ionized calcium levels, however, can be labor intensive and may not be readily available in the outpatient setting. Correcting for hypoalbuminemia eliminates the major difference that may occur between total serum calcium and ionized calcium.
Second, exclusion of secondary causes of hyperparathyroidism is essential to the diagnosis of NPHPT. The most important secondary causes are listed in Table 18.2.
Table 18.2
Causes of secondary hyperparathyroidism
Causes of secondary hyperparathyroidism |
|---|
Hypovitaminosis D |
Decreased glomerular filtration rate |
Thyroid disease |
Hypercalciuria |
Malabsorptive diseases (e.g., celiac disease) |
Liver and pancreatic diseases |
Other bone diseases (e.g., Paget’s disease of bone) |
Medications (e.g., thiazide diuretics, lithium, etc.) |
Most notably, serum 25-hydroxyvitamin D levels below 30 ng/mL may cause increased PTH levels. Whereas some authors have used a cut point of 20 ng/mL to exclude hypovitaminosis D as a driving force for increased PTH levels, other authors have demonstrated that 25-hydroxyvitamin D levels between 20 and 30 may affect PTH levels [7, 8]. Furthermore, PTH levels have been shown to begin to rise when GFR falls below 60 mL/min and continue to increase as kidney function worsens [9, 10]. Several medications have also been associated with elevated PTH levels, most notably thiazide diuretics and lithium, but also estrogens, bisphosphonates, denosumab, and anticonvulsants [4].
Initial observations of NPHPT were based on populations referred to metabolic bone clinics for the evaluation of low bone mass and therefore tended to overestimate disease prevalence. Several attempts have been made to evaluate the prevalence of NPHPT in population-based cohorts. Reproducibility and generalizability of these numbers, however, appear to be limited as studies have tended to differ in gender distribution, geographic location, and efforts to exclude cases of secondary hyperparathyroidism.
In a survey of 5202 postmenopausal women in Sweden between the ages of 55 and 75, an estimated 0.5 % had elevated PTH levels with normal serum calcium values. Although this cohort did not exclude secondary etiologies of hyperparathyroidism, most cases of NPHPT were confirmed by surgical pathology [11].
Based on the NHANES data, Misra et al. found that 1 % of men and women aged greater than 45 years had PTH levels of 65 pg/mL or higher in addition to normal albumin-corrected serum calcium values. Notably, these authors excluded subjects with GFR <60 mL/min and 25-hydroxyvitamin D levels <30 ng/dL [12].
In a separate study, 6 of 100 postmenopausal women surveyed in Spain had NPHPT after excluding renal disease, vitamin D insufficiency (<30 pg/dL), and malnutrition [13].
In the Osteoporotic Fractures in Men (MrOS) population of men aged 65 years or older, the prevalence of NPHPT was 0.4 %. Separately, the Dallas Heart Study of men and women aged 18–65 years determined an initial prevalence of NPHPT of 3.1 %, a prevalence which decreased to 0.6 % after 8 years of follow-up, with some subjects having normalization of PTH levels, while others had progression to overt hypercalcemia [2].
The highest prevalence reported to date is 16.7 % as determined in the Canadian Multicentre Osteoporosis Study (CaMos) [14]. Of note, however, that study did not exclude patients with secondary hyperparathyroidism and used a lower limit of 25-hydroxyvitamin D levels of 20 ng/dL. Most recently, a survey of residents from a small village in Southern Italy found that 0.44 % of participants had NPHPT [15].
Gender predominance is also difficult to assess, as most cohorts studied to date have been gender specific. There does seem, however, to be a preponderance of NPHPT in the female gender [3, 16].
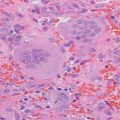
The histopathology of NPHPT follows other subgroups of PHPT: surgical resection has identified that most cases represent a single adenoma (80 %) followed by multiglandular hyperplasia (20 %) [11, 17]. A single case of carcinoma has been reported to date [18].
As noted previously, most studies that have evaluated subjects with NPHPT have included primarily referral populations, with most patients having symptoms related to their metabolic disease. Most notably, 25–57 % of subjects have osteoporosis on initial evaluation (either by bone mineral density (BMD) criteria or due to a history of fragility fractures), with 9–25 % having a history of nephrolithiasis [16, 17, 19–22].
In contrast to patients with asymptomatic mild PHPT in whom cortical bone loss occurs preferentially, osteoporosis in patients with NPHPT appears to be more common at the lumbar spine (34–66 %) and hip (38–47 %), with comparatively preserved bone mineral density at the distal one-third radius [16, 19, 23].
In a New York population-based cohort, up to 40 % of patients with NPHPT developed clinical features of PHPT including hypercalcemia, hypercalciuria, and/or significant bone loss during a median 3-year follow-up period [19, 22]. Patients who developed hypercalcemia on follow-up tended to be older and were more likely to have higher baseline values for serum calcium and urinary calcium [2, 19]. Such observations suggest the presence of an asymptomatic form of NPHPT that may only be identified in population-based screening. This is consistent with the dual-phase hypothesis proposed by Rao et al. in 1988 which suggested that the first biochemical abnormality of PHPT may be an elevated PTH level which is considered subclinical in the setting of a normal serum calcium value [24], a concept recently supported by Cusano et al. [2]. Hyperparathyroidism would thus be viewed as a spectrum that encompasses both normocalcemia and hypercalcemia and in parallel with, but not necessarily linked to, silent and symptomatic disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree