Normal Anatomy and Histology
GROSS ANATOMY
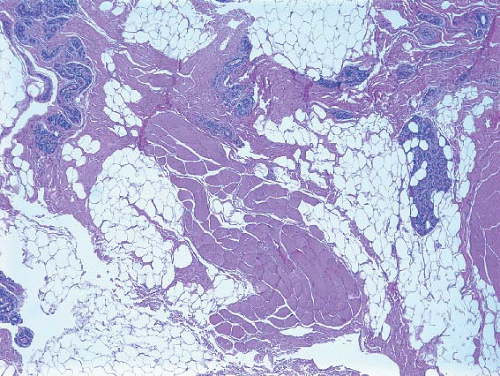
The breast lies on the anterior chest wall over the pectoralis major muscle and typically extends from the second to the sixth rib in the vertical axis and from the sternal edge to the mid-axillary line in the horizontal axis. Breast tissue also projects into the axilla as the tail of Spence. The breast extends laterally over the serratus anterior muscle and inferiorly over the external oblique muscle and the superior rectus sheath. Bundles of dense fibrous connective tissue, the suspensory ligaments of Cooper, extend from the skin to the pectoral fascia and provide support to the breast. The only boundary of the breast that is anatomically well-defined is the deep surface where it abuts the pectoral fascia. However, despite this macroscopic demarcation, microscopic foci of glandular tissue may extend into and even through the pectoral fascia (Fig. 1.1, e-Fig. 1.1). The clinical importance of this observation is that even a total mastectomy does not result in the removal of all glandular breast tissue.
The principal arterial supply to the breast is provided by the internal mammary and lateral thoracic arteries. Branches of the thoracoacromial, intercostal, subscapular, and thoracodorsal arteries make minor contributions to the mammary blood supply.1 Venous drainage of the breast, as in other locations, shows considerable individual variation but largely follows the arterial system. The most important drainage basin for lymphatic flow from the breast is the axilla, and the axillary lymph nodes receive >90% of the lymph drained. A small portion of lymph drains via the internal thoracic and posterior intercostal lymphatics into the internal mammary and posterior intercostal lymph nodes, respectively.
HISTOLOGY
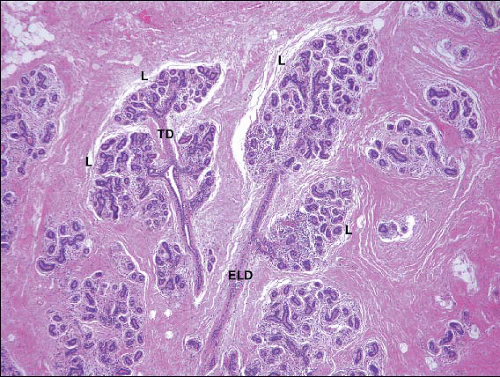
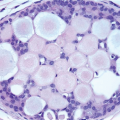
The adult female breast consists of a series of branching ducts and ductules that terminate in the acini (also called terminal ductules). The acini are grouped together to form the lobules. The arrangement of these structures has been likened to a flowering tree (Fig. 1.2, e-Fig. 1.2).2 The lobules represent the flowers, draining into ductules and ducts (twigs and branches) which, in turn, drain into the collecting ducts (trunk) that open onto the surface of the nipple. At the nipple, the ducts are expanded to form lactiferous sinuses. The sinuses terminate in cone-shaped ampullae just below the surface of the nipple.
The mammary ducts and lobules are embedded within a stroma composed of varying amounts of fibrous tissue and adipose tissue. The stroma comprises the major portion of the non-lactating adult breast, and the relative proportions of fibrous tissue and adipose tissue vary with age and among individuals (Fig. 1.3, e-Fig. 1.3).
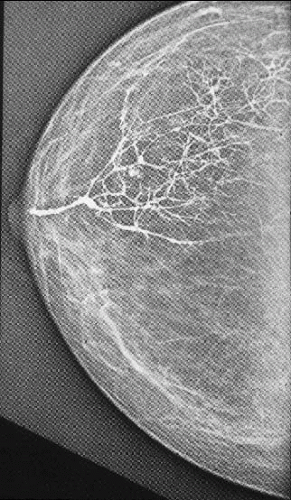
The ductal-lobular system of the breast is arranged in the form of segments or lobes. Although these segments can be readily appreciated by injecting the ductal system with dyes or radiologic contrast agents (Fig. 1.4), they are anatomically poorly defined, and no obvious bound aries can be appreciated between these segments during surgery, upon gross inspection of mastectomy specimens, or on histologic examination. In addition, the segments show considerable individual variation with regard to their distribution,3 and the ramifications of individual segments may overlap. The segmental nature of some neoplastic processes in the breast,
particularly ductal carcinoma in situ, is now widely appreciated. This recognition, in conjunction with observations in developmental anatomy and morphology, has led to the development of the “sick lobe” hypothesis of breast cancer.4,5 This theory postulates that early breast carcinoma (ductal carcinoma in situ) is a lobar disease, often isolated to a single ductal system (or lobe). Thus, surgical resection of the involved segment is an important therapeutic goal. Unfortunately, because it is not possible for the surgeon to define the boundaries of the involved segment intraoperatively, performing a “segmentectomy” to remove the entirety of a diseased segment is at this time more of a theoretical concept than a practically attainable goal.
particularly ductal carcinoma in situ, is now widely appreciated. This recognition, in conjunction with observations in developmental anatomy and morphology, has led to the development of the “sick lobe” hypothesis of breast cancer.4,5 This theory postulates that early breast carcinoma (ductal carcinoma in situ) is a lobar disease, often isolated to a single ductal system (or lobe). Thus, surgical resection of the involved segment is an important therapeutic goal. Unfortunately, because it is not possible for the surgeon to define the boundaries of the involved segment intraoperatively, performing a “segmentectomy” to remove the entirety of a diseased segment is at this time more of a theoretical concept than a practically attainable goal.
The number of segments in the breast and their relationship to each other has long been a matter of debate. Most textbooks indicate that there are 15 to 20 ductal orifices on the nipple’s surface and suggest that this corresponds to the number of ductal systems, segments, or lobes in the breast.6, 7, 8 and 9 However, the relationship between the number of nipple duct orifices, nipple ducts, and breast segments remains unclear. One study indicated that there are more orifices on the surface of the nipple than there are nipple ducts or breast segments. The proposed explanations for this are: 1) that some orifices on the nipple surface represent openings of sebaceous glands or other non-ductal structures that do not contribute to the ductal-lobular anatomy of the breast and 2) that some lactiferous ducts bifurcate immediately prior to entering the nipple.6 In contrast, a more recent study that included three-dimensional reconstruction found that the number of orifices on the surface of the nipple is fewer than the
number of nipple ducts because multiple ducts may join to share a common orifice.10 Further, a number of mammary duct injection studies have suggested that there are far fewer (only between 5 and 10) discrete breast ductal systems or segments than there are ducts in the nipple or orifices on the nipple surface.
number of nipple ducts because multiple ducts may join to share a common orifice.10 Further, a number of mammary duct injection studies have suggested that there are far fewer (only between 5 and 10) discrete breast ductal systems or segments than there are ducts in the nipple or orifices on the nipple surface.
The issue of anastomoses between ductal systems is also unresolved. One study indicated that while ductal systems may lie in close proximity to one another and even intertwine within a particular quadrant, they do not interconnect.6 However, anastomoses between ductal systems have been reported by others.11
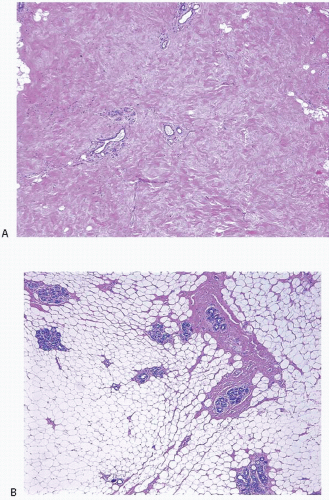
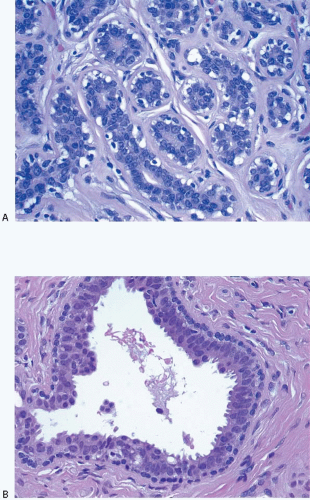
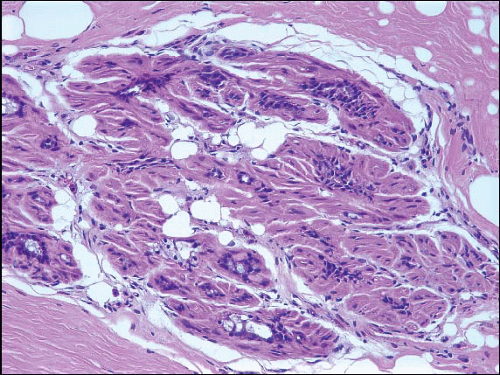
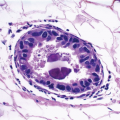
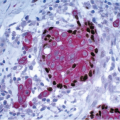
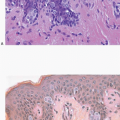
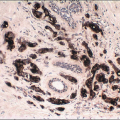
The cellular lining throughout the ductal-lobular system is bilayered. It consists of an inner (luminal) epithelial cell layer and an outer (basal) myoepithelial cell layer. The importance of this double cell layer cannot be overemphasized, because it is one of the main guides used to distinguish benign from malignant lesions.12 The luminal epithelial cells of the resting breast ducts and lobules are cuboidal to columnar in shape and typically have pale eosinophilic cytoplasm and relatively uniform oval nuclei. These epithelial cells express a variety of low-molecular-weight cytokeratins, including cytokeratins 7, 8, 18, and 19. The outer or myoepithelial cell layer, although always present, is variably distinctive (Fig. 1.5, e-Fig. 1.4). Myoepithelial cells range in appearance from barely discernible, flattened cells with compressed nuclei to prominent epithelioid cells with abundant clear cytoplasm (Fig. 1.6, e-Fig. 1.5). In some cases, the myoepithelial cells have a myoid appearance featuring a spindle cell shape and dense, eosinophilic cytoplasm, reminiscent of smooth muscle cells (Fig. 1.7, e-Fig. 1.6). Even when inconspicuous on hematoxylin and eosin-stained sections, myoepithelial cells can be readily demonstrated using immunohistochemical stains for a variety of markers including S-100 protein, actin, calponin, smooth muscle myosin heavy chain, p63, and CD10 (Fig. 1.8). However, these markers vary in both sensitivity and specificity for myoepithelium. Myoepithelial cells also express high-molecular-weight cytokeratins 5/6, 14, and 17 (Fig. 1.9), but the expression of cytokeratin 14 is restricted to the myoepithelial cells of the large ducts and terminal ducts; expression is not seen in myoepithelial cells of the intralobular ductules and acini.13
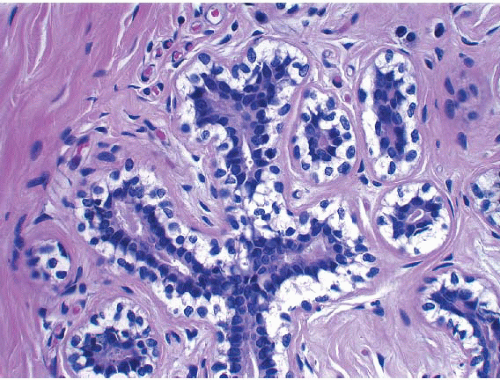 FIGURE 1.6 Myoepithelial cells can vary in their histologic appearance. Myoepithelial cells in this lobule show prominent cytoplasmic clearing. |
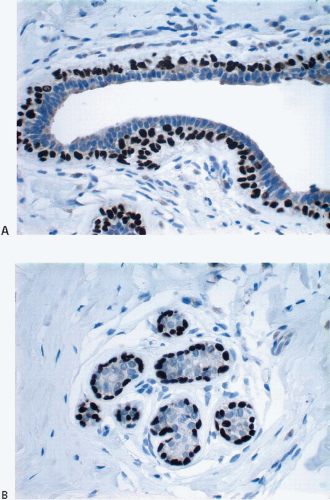 FIGURE 1.8 Extralobular duct (A) and lobule (B) immunostained for p63. The myoepithelial cells show strong nuclear reactivity, whereas the epithelial cell nuclei are negative. |
A third cell type dispersed irregularly throughout the ductal-lobular system expresses high-molecular-weight cytokeratins 5 and 14 in the
absence of expression of markers of differentiated luminal epithelial cells (such as low-molecular-weight cytokeratins) or myoepithelial cells (such as smooth muscle actin). These cells have been postulated to represent progenitor cells capable of differentiating into both luminal epithelial cells and myoepithelial cells.14 The relationship of these putative “ progenitor” cells to mammary stem cells is at this time an unresolved issue.14,15 However, it is now clear that cells with the characteristic features of stem cells (i.e., self-renewal and the ability to differentiate down different cell lineages to form all of the cell types found in the mature tissue) do exist in the breast. Mammary stem cells appear to be important in both breast development16 and mammary carcinogenesis.15,17,18 Immunophenotypic features that have been a ssociated with the mammary stem cell population include lack of expression of estrogen receptor (ER) and progesterone receptor (PR), high expression of CD44, low or absent expression of CD24, and expression of aldehyde dehydrogenase (ALDH1),18,19 although the most accurate combination of markers to reliably identify mammary stem cells is unresolved at this time.
absence of expression of markers of differentiated luminal epithelial cells (such as low-molecular-weight cytokeratins) or myoepithelial cells (such as smooth muscle actin). These cells have been postulated to represent progenitor cells capable of differentiating into both luminal epithelial cells and myoepithelial cells.14 The relationship of these putative “ progenitor” cells to mammary stem cells is at this time an unresolved issue.14,15 However, it is now clear that cells with the characteristic features of stem cells (i.e., self-renewal and the ability to differentiate down different cell lineages to form all of the cell types found in the mature tissue) do exist in the breast. Mammary stem cells appear to be important in both breast development16 and mammary carcinogenesis.15,17,18 Immunophenotypic features that have been a ssociated with the mammary stem cell population include lack of expression of estrogen receptor (ER) and progesterone receptor (PR), high expression of CD44, low or absent expression of CD24, and expression of aldehyde dehydrogenase (ALDH1),18,19 although the most accurate combination of markers to reliably identify mammary stem cells is unresolved at this time.
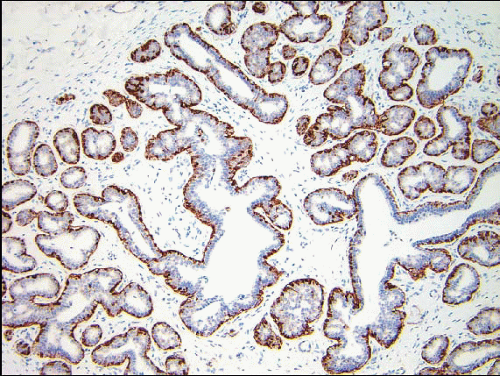 FIGURE 1.9 Immunostain for the basal cytokeratin CK5/6 highlighting the myoepithelial cells around ductules and acini. |
A basal lamina consisting of type IV collagen and laminin surrounds the mammary ducts, ductules, and acini.20,21




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree