Nonmelanoma Skin Cancer
Malcolm A. Buchanan
Carsten E. Palme
Faruque Riffat
Michael J. Veness
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide and is managed by a diverse group of clinicians, including primary care physicians, dermatologists, otolaryngology—head and neck surgeons, surgical oncologists, plastic and reconstructive surgeons, and radiation oncologists. It is important that treating clinicians have a clear understanding of the epidemiology, staging, management, and prognosis of this disease. NMSC is a heterogeneous group of malignancies encompassing many different histologic subtypes, requiring different management approaches, and with widely varying prognoses.1 These malignancies range from ubiquitous lesions, such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), to relatively uncommon lesions, including Merkel cell carcinoma (MCC), adnexal carcinomas, and mesenchymal tumors, such as cutaneous angiosarcoma and Kaposi sarcoma (KS).
EPIDEMIOLOGY
The most common NMSC is BCC, which constitutes 70% to 75% of cases, followed by SCC (20% to 25%).2 MCC constitutes <5% of NMSC, and other histologic subtypes make up the remainder.3,4 In general, males are affected more often than females, and the incidence increases with age.
The prevalence of NMSC has reached epidemic rates in regions such as Australia, where the incidence of skin cancer is the highest in the world.2 Worldwide, there are differing rates of NMSC in various populations, but in most regions, the incidence is increasing.2,5,6
In Australia, the incidence of NMSC has risen significantly from 1985 to 2002, with the annual incidence of BCC increasing from 657/100,000 to 884/100,000, and SCC more than doubling from 166/100,000 to 387/100,000.2 This continued increase has occurred despite recent public health campaigns, such as “SunSmart,” which advocates protective clothing, sun protection factor in excess of 30+ sunscreen, and avoidance of excessive sunlight, especially during peak sunlight hours.7 In 2002, the incidence of NMSC in Australia was more than 5 times that of all other cancers, combined with an annual rate of 1,170/100,000.2 In the United States, over 1 million new cases of NMSC are diagnosed annually.8
Death from NMSC is low compared with other cancers. Between 1998 and 2005, there was an average of 382 deaths per year in Australia, ˜1 to 3 per 100,000.9 Overall, NMSC has a good prognosis, with a low recurrence rate and >90% 5-year disease-specific survival rates. Despite this, NMSC, and in particular a subset of patients with high-risk SCC, can still provide a challenge for both patient and clinician, due to the development of locally recurrent disease in ˜10% and the propensity for metastasis to the neck in 3% to 5% of patients.10,11,12,13 Patients diagnosed with either an SCC or an MCC are at risk of developing metastasis and dying of their disease. Death resulting from BCC is extremely rare.
ETIOLOGY
Ultraviolet Radiation
Environmental exposure to ultraviolet (UV) radiation is the major etiologic factor that damages DNA and leads to the development of NMSC. The pathogenesis of SCC strongly correlates with cumulative exposure of UV radiation, in particular, UVB (290 to 320 nm). The sun-exposed head and neck is the region of the body most frequently affected by NMSC.3 Risk factors include increasing age (particularly age over 70 years2) male gender, Caucasian ethnicity, chronic sun exposure, outdoor occupation, acquired or inherited immunosuppression, and certain rare systemic diseases such as epidermolysis bullosa, oculocutaneous albinism, and xeroderma pigmentosum (XP).1,14,15,16 Additional factors such as proximity to the equator, ozone layer depletion, and both occupational and recreational exposure significantly increase this risk.15 NMSC is rare in dark-skinned races due to the protective role of melanin in UV-induced damage of skin cells.17
Immunosuppression
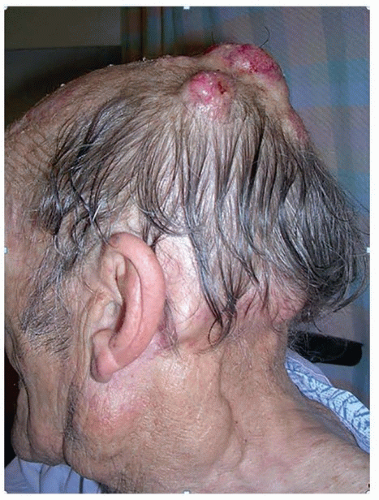
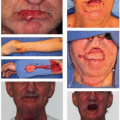
Immunosuppression such as in solid organ transplantation, hematologic malignancies, including non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) (Fig. 8.1) and human immunodeficiency virus (HIV) infection increases the risk of NMSC. Posttransplantation patients and those with autoimmune disorders, or on immunosuppressive agents, exhibit an increased risk, particularly of SCC, with increasing risk associated with increasing duration of immunosuppression.18,19 Recipients of solid organ transplants exhibit approximately a 100-fold increase in the risk of developing SCC compared with a 10- to16-fold increase in risk of developing BCC.20 The risk for developing cervical lymph node metastases lies between 10% and 18%,21,22 and the risk of death is significantly increased in patients who develop SCC after a renal transplant.23
Treatment-related immunosuppression, which classically occurs in solid organ transplant recipients, results in an 18 to 250 times increased risk of SCC24,25 and, to a lesser extent, BCC. Furthermore, there is a greater risk of developing metastasis involving both regional and distant sites.26 In the Australian transplant population, at least 45% of patients developed an SCC within 10 years of transplantation, whereas patients from Europe had an incidence of 10% to 15% at 10 years following
transplant.27 In the renal transplant population in Queensland, Australia, the incidence of NMSC at 20 years was 81%, with 75% having invasive SCC.28
transplant.27 In the renal transplant population in Queensland, Australia, the incidence of NMSC at 20 years was 81%, with 75% having invasive SCC.28
Immunosuppression secondary to HIV has a different effect on NMSC. Associated with this greater incidence is a propensity for aggressive SCC, and an increased rate of spread to regional lymph nodes. Unlike other forms of immunosuppression, there does not appear to be an alteration in the ratio of BCC to SCC. Currently, it is unclear if CD4 count or HIV viral load affects the development of NMSC in HIV patients.29 There are current data to support a role for human papillomavirus (HPV) in the development of SCC. This association has been demonstrated in the immunocompromised population, where up to 90% of tumors contain HPV DNA.30
Gene Mutations and Inherited Conditions
As with all malignancies, acquired and inherited mutations are involved in the pathogenesis of NMSC. The p53 tumor suppressor gene has an important regulatory role in the cell cycle, as well as in DNA repair and apoptosis.31 Mutations of this gene are implicated in the etiology of many cancers, including SCC and BCC. Alterations in pyrimidine dimers, induced by UV radiation, may inactivate this gene, causing dysregulation of the cell cycle, failure of apoptosis, and tumor formation.32 Another gene relevant in the pathogenesis of BCC is patched (PTCH1), located on chromosome 9, which was first identified in individuals with the autosomal dominant BCC nevus (Gorlin) syndrome.33 Patients with this syndrome have defects in the sonic hedgehog signaling pathway and present with multiple BCCs, odontogenic cysts, skeletal defects, palmar and plantar pits, and calcification of the falx cerebri.33
Other inherited conditions, including the autosomal recessive XP, can predispose individuals to a 100-fold increase in the risk of NMSC. In this condition, cells have an impaired ability to repair UV-induced damage, leading to multiple SCC and other skin cancers. The mutation involved disrupts the nucleotide excision repair, which enzymatically repairs UV-induced DNA damage.31 Epidermodysplasia verruciformis is another autosomal recessive condition, in which increased susceptibility to viral oncogenesis, secondary to HPV infection, results in widespread wart formation followed by the appearance of cutaneous SCC.34
Cigarette Smoking and Carcinogens
Cigarette smoking has been implicated in the development of many malignancies, although its role in the development of NMSC is controversial. A Dutch study showed a doubling of the risk of development of cutaneous SCC in smokers with an associated dose response.35 Some studies have supported the association, quoting increased rates of 1.5 to 4 times, whereas other studies have found no association. In contrast to the Dutch study, a large prospective study involving over 300,000 construction workers in Sweden did not find any association between smoking and the development of SCC.36
Other risk factors include exposure to chemical carcinogens, such as arsenic.37 Arsenic is associated strongly with the development of a variety of dermatologic manifestations and malignancies, including SCC and BCC. Areas of chronic irritation and scarring also predispose to SCC, which is known to arise in chronic “Marjolin” ulcers, sinus tracts, and scars.33
Precursor Lesions
Actinic keratosis (AK) is a dysplastic keratinocytic lesion arising within the epidermal layer of the skin, induced by UV radiation. \\14,38 In Australia, up to 50% of people over 40 years of age have one or more AK.14 Although a substantial proportion (25% to 75%) of AK regress over time, a number eventually progress to invasive SCC. Estimates of the rate of progression of an individual AK to SCC have been reported at up to 20%. Despite this, two longitudinal studies have reported a considerably lower annual rate of transformation—0.096% per year14; and 0.60% at 1 year and 2.57% at 4 years.38 These studies reported that 60% to 65% of SCC arose from preexisting AK. Although the chance of an individual AK transforming into invasive SCC is low, individuals at risk usually have multiple AK, with one study documenting an average of 46.
BASAL CELL CARCINOMA
Clinical Presentation
Unlike SCC, BCC arises de novo, with no obvious precursor lesions. The nodular subtype is the most common and accounts for ˜60% of BCC. Other variants are superficial or infiltrative. Nodular and morpheaform are the most common subtypes in the head and neck region; superficial BCCs commonly occur on the trunk.39
Nodular BCC typically presents as a “pearly” telangiectatic nodule with rolled borders. Central ulceration, crusting,
and bleeding may occur. Superficial BCC may present as an asymptomatic plaque or papule and is pink/red in color. Most are asymptomatic, with ulceration, itching, and bleeding being uncommon. Morphoeic lesions are smooth, flesh-colored plaques or papules resembling scars with ill-defined borders.40 They are often long-standing asymptomatic lesions and may be deeply invasive by the time of diagnosis. Perineural invasion (PNI) is frequently present. In the head and neck, the nose is the site most commonly affected, followed by other sun-exposed areas, such as the scalp and ear.41,42 Prospectively acquired data in Australia have shown that 57% (379 out of 663) of BCCs were located in the head and neck, with the nose, cheek, forehead, and ears most commonly affected.43 BCC rarely spreads to involve regional lymph nodes.
and bleeding may occur. Superficial BCC may present as an asymptomatic plaque or papule and is pink/red in color. Most are asymptomatic, with ulceration, itching, and bleeding being uncommon. Morphoeic lesions are smooth, flesh-colored plaques or papules resembling scars with ill-defined borders.40 They are often long-standing asymptomatic lesions and may be deeply invasive by the time of diagnosis. Perineural invasion (PNI) is frequently present. In the head and neck, the nose is the site most commonly affected, followed by other sun-exposed areas, such as the scalp and ear.41,42 Prospectively acquired data in Australia have shown that 57% (379 out of 663) of BCCs were located in the head and neck, with the nose, cheek, forehead, and ears most commonly affected.43 BCC rarely spreads to involve regional lymph nodes.
Histology
BCC is typified by collections of cells resembling the basal layer of the epithelium. Retraction between the stroma and tumor may be present as an artifact and helps to differentiate BCC from appendageal tumors of similar appearance.44 Morphoeic BCC differs histologically from the other subtypes of BCC as the stroma contains little mucin and retraction artifact is rare. PNI does occur but is uncommon.
Management
A punch or incisional biopsy of the lesion is required for initial diagnosis. As BCCs rarely metastasize, staging investigations are unnecessary, but computed tomography (CT) scans of the head and/or neck are performed in cases of locally advanced cancer to assess depth of invasion and involvement of functionally important soft tissue and bony structures (i.e., parotid gland (PG), external auditory canal, petrous temporal bone).
Surgical
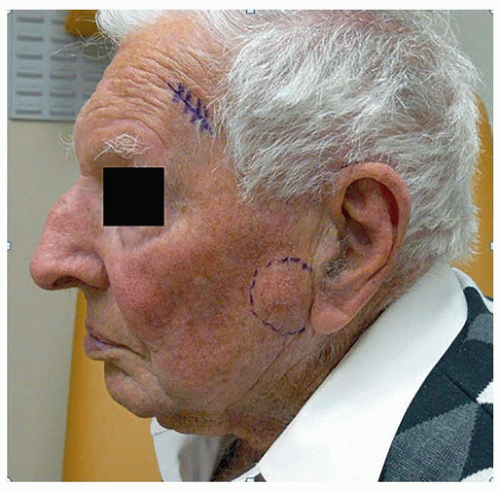
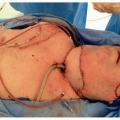
Lesions are excised, typically with a margin of 3 to 5 mm. The majority of lesions can be excised and closed primarily. In patients in whom primary closure is not feasible, local flaps and skin grafts are used (Fig. 8.2). A positive margin has been reported to be associated with a 30% to 40% local recurrence rate,45 and in these cases, patients should be considered for further surgery or adjuvant radiotherapy (RT). Positive margins underlying local flaps should rarely be left untreated because of the risk of undetected and delayed deep recurrence. Selected patients, such as those with morphoeic or recurrent BCC, can be referred for Mohs micrographic surgery, a technique in which serial sections of skin are excised and the peripheral and deep margins examined, so that 100% of the surgical margins are evaluated. Patients with morphoeic and large BCCs require wider surgical margins to maximize the chance of complete resection. For primary morphoeic lesions, the rate of complete excision with increasing peripheral surgical margins is 3-mm margin, 66%; 5-mm margin, 82%; 13- to 15-mm margin >95%.46
Nonsurgical
A variety of nonsurgical options are also available to the clinician, including RT, cryosurgery, photodynamic therapy, curettage and cautery, topical treatment, and intralesional injection.
Only a few randomized controlled trials have reported on the outcome of RT on patients with BCC. A Cochrane review suggested that either RT or surgery results in the lowest recurrence rates.47 A trial of 347 patients examining RT versus excision of facial BCCs of <40 mm in diameter demonstrated fewer recurrences in the surgical cohort at 4 years (RR 0.09), and that cosmetic outcome was enhanced postsurgery (87% rated as “good”) at 4 years compared with RT (69%).48 Conversely, in a separate trial of 374 patients, no significant difference was seen in recurrence rates between patients receiving RT or Mohs surgery, and overall cosmetic outcome did not differ between treatment groups.49 RT is an effective option if surgery is declined or the outcome (form and/or function) is likely to be better nonsurgically.
Adjuvant RT is an option in the setting of close or positive excision margins, especially if a flap has been used for reconstruction, as detecting deep recurrence, especially in the midface, can be difficult. Up to 30% of incompletely excised BCCs will recur locally,50 making RT a useful modality, especially if reexcision is not an option. In a trial of adjuvant RT versus surgery alone, RT improved the 5-year local control rate from 61% to 91%.51 Ten-year local control rates were similar between the two groups (92% vs. 90%), indicating that most local recurrences can be salvaged surgically, although some patients require reconstruction after wide local excision. Patients with infiltrative BCC, particularly those with PNI, should be considered for adjuvant RT, given the propensity of these tumors to recur locally or spread to the skull base through perineural pathways.
Patients with XP should not undergo RT especially at a younger age because of the risk of inducing skin cancers. Similarly, lower limb lesions, especially in older patients suffering from diabetes and peripheral vascular disease, should not be irradiated if possible, because of the risk of delayed wound healing.52
SQUAMOUS CELL CARCINOMA
Clinical Presentation
Morphologically, the appearance of SCC exhibits a range of phenotypic variation. Classically, the appearance is of a shallow ulcer with raised, indistinct borders. A plaque often covers the lesion. Similar to BCC, sun-exposed areas of the head and neck are most commonly affected, with involvement of the lip or ear associated with a poorer prognosis and increased risk of metastases. Local symptoms and signs, such as numbness, pain, trismus, tumor immobility, paresthesias, dysesthesias, and cranial nerve palsy, are signs of advanced disease and may indicate deep tissue invasion and/or underlying perineural spread, which are poor prognostic factors.16
Patients with SCC may have a history of premalignant lesions, most commonly AK. Invasive SCC may also occur in the absence of a history of premalignant lesions. Bowen disease (intraepithelial SCC) presents as well-demarcated, erythematous, scaly keratotic papules and plaques.53 The presence of nodal metastases has a potential adverse impact on prognosis in terms of morbidity, mortality, and quality of life.54 Distant spread is rare but may occur in more advanced, neglected, and/or recurrent cancers. Lung and bone are reported to be the most common sites of distant metastasis,55,56 with the liver and brain also potentially affected.16 In a study of 122 patients treated for metastatic SCC, 7% developed distant metastatic disease, with the lung the most common site.56
Histology
SCC and its precursor lesions (AK and Bowen disease) are characterized by sheets and ridges of squamous cells. AK involves only part of the epidermis, Bowen disease involves the full thickness of the epidermis, and invasive SCC spreads beyond the basement membrane. SCC arises from the keratinocytes of the spinous layer of the epidermis. There is infiltration of the dermis by atypical squamous cells surrounded by an inflammatory infiltrate. The degree of cellular differentiation is categorized as mild, moderate, or severe and is of prognostic value. The degree of differentiation correlates with the extent of keratinization, nuclear hyperchromasia, and increased mitotic activity.44 The more poorly differentiated the tumor, the fewer keratin pearls are present. The depth of anatomic invasion is indicated by Clark’s level staging system57:
Level 1: SCC confined to the epidermis (SCC in situ)
Level 2: Invasion into the papillary dermis
Level 3: Invasion to the junction of the papillary and reticular dermis
Level 4: Invasion into the reticular dermis
Level 5: Invasion into the subcutaneous adipose tissue
Metastasis to the Neck
The risk of developing lymph node metastasis in patients with SCC is uncommon, and ˜3% to 5%, but increases in patients with unfavorable primary tumor features, that is, high-risk SCC.11,12,13 Patients developing local recurrence are at higher risk of lymph node metastasis.10,12,58 The parotid gland (PG) is the most frequent site for metastasis in patients with head and neck primaries. Patient factors that predict the development of metastasis include male gender, immunosuppression, and delayed presentation.11,59 Tumor factors include histologic grade (poorly differentiated or undifferentiated), size (>2 cm), depth/thickness (>4 mm), invasion of adjacent tissue, anatomic location (ear, lower lip, and cheek), presence of perineural and/or lymphovascular invasion (LVI), and growth rate. Over 70% of lymph node metastasis presents within 1 year of treatment of the primary lesion, whereas few patients present with lymph node metastasis after 5 years.12
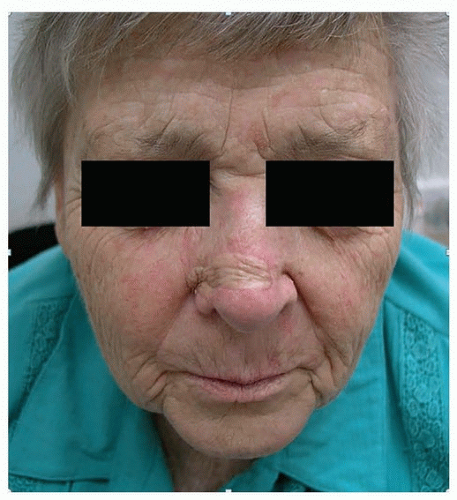
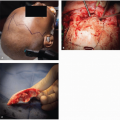
Regional nodes can be separated broadly into two groups, namely, parotid (preauricular and parotid tail) and cervical nodes (levels I to V). The location of a primary cutaneous SCC is an important determinant of the site of potential lymph node metastasis. The most frequent location for such a lesion is the lateral aspect of the head (Fig. 8.3). Metastasis is most commonly identified in parotid, level II (i.e., jugulodigastric), and external jugular chain nodes. Parotid nodes represent the first echelon of lymphatic drainage from the face, forehead, anterior scalp, temple, and ear. In Australia, metastatic cutaneous SCC is the most common malignant neoplasm of the parotid.60 Facial lesions tend to metastasize to level I and II cervical nodes, whereas anterior lesions of the scalp, ear, temple, and forehead usually metastasize to parotid +/- level II cervical lymph nodes.61 Drainage to multiple first echelon nodes is common. Drainage to contralateral nodes occurs in 10% of patients, predominantly in those with midline cancers.62 Cancers posterior to the tragus usually metastasize to level V or the occipital nodes.
High-Risk Tumor Features
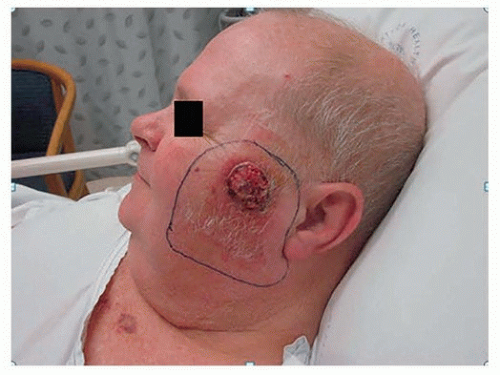
Tumor size, using a cutoff of 2 cm (Fig. 8.4), is associated with a significant difference in the rate of lymph node metastasis.10,11,63 There is, however, a limitation in applying two-dimensional tumor size as a sole prognostic factor. In a study of 266 patients with metastatic lymph node metastasis, where 70% of lesions were <2 cm in size, tumor thickness was >4 mm in the majority of patients with T1 lesions, all of whom had metastasis.58 There was a significant correlation between increasing thickness of the cancer and size of the lesion, suggesting that these cancers had a propensity for both vertical and horizontal growth. It was noted that not all large SCC metastasized, inferring that
lesions that are horizontally large (2 to 3 cm), but not thick (i.e., 2 to 3 mm), may lack the tendency to metastasize.
lesions that are horizontally large (2 to 3 cm), but not thick (i.e., 2 to 3 mm), may lack the tendency to metastasize.
Thickness of the cancer is also of prognostic importance.12 Thickness of the cancer >6 mm was a highly significant independent predictor for the development of metastases in a large German prospective study.12 Fourteen of 90 patients with cancers thicker than 6 mm developed regional metastasis, whereas no patients with cancers thinner than 2 mm developed regional metastasis. Another study demonstrated that although only one-third of patients with SCC have lesions >4 mm thick, these accounted for >80% of cancers developing metastatic nodal disease.22 In a study involving more than 500 patients, no patients with an SCC of thickness <2 mm developed metastasis, whereas approximately 20% of patients with a lesion >5 mm developed regional nodal metastases.64 In keeping with thickness, Clark levels have also been investigated and reported to be predictive. SCC measuring <4 mm thick or Clark level I to III had a metastatic rate of 6.7%, whereas the rate for SCC > 4 mm thick or Clark level IV or V was 45.7%.10
Desmoplastic SCC is an aggressive histologic variant, most frequently found on the ears, nose, and forehead. It is characterized by the presence of PNI, an invasive clinical course, and poor prognosis.65 Patients with desmoplastic SCC have 10 times the risk of local recurrence and 6 times the risk of metastasis compared with other SCC subtypes.64 Desmoplasia is reported to be the most important histologic feature for local recurrence, with 24% of 51 patients with desmoplasia versus 1% of 564 patients without desmoplasia developing local recurrence.12
Recurrent cancers are associated with a marked increase in the risk of developing metastases to the neck. Patients with inadequately excised SCC are at risk of both local recurrence and the development of nodal metastasis. The risk of nodal metastasis has been shown to be 15% in patients with recurrent lip SCC, compared with 2% in those with de novo lesions.10 The incidence of lymph node metastasis was 32% and 45% in the setting of recurrent lip and ear SCC, respectively.58 In one study, 18% of 78 patients had metastatic cancer following tumor recurrence (HR 2.81).66 In a review of 122 patients with metastatic SCC, 11% of patients had lesions that were recurrent.56
Poorly differentiated SCC is more likely to be associated with the development of regional metastases. A significant difference in the rate of nodal metastasis between high- and lowgrade SCCs (17% vs. 4%) has been reported.67 Other studies have demonstrated a difference in tumor behavior, based on histologic grade, with the rate of poorly differentiated SCC increased in patients developing metastasis (44% vs. 5%).63 An Australian study also supported this finding, with 46% of patients with nodal metastasis displaying moderate or poor differentiation of their primary SCC, compared with 12% with a well-differentiated grade.58
Perineural invasion (PNI) refers to tumor growth in or around a nerve68 and occurs by the contiguous spread of malignant cells along the potential space between a nerve and its surrounding sheath. PNI occurs in <5% of all cutaneous malignancies and is more common among SCC, involving 2.5% to 14% of cases, compared with 0.18% to 10% of BCC.69 The presence of PNI is significant in that it confers an increased risk of recurrence in both BCC and SCC and the development of metastasis in SCC, and a poorer prognosis due to more aggressive tumor behavior.69 The risk of death from PNI is much less likely with BCC.
PNI can be broadly classified as either “incidental” or “clinical.” Incidental PNI is identified only at histopathology in clinically asymptomatic patients with negative imaging. Other terms used in the literature to describe incidental PNI include “minimal” or “microscopic” PNI. PNI is classified as “clinical” when the patient exhibits sensory or motor changes or there is radiographic evidence of perineural spread within a named nerve. It may also be referred to as “extensive” or “macroscopic” PNI.
The distinction between incidental and clinical PNI is prognostic. One study reported a 5-year local control rate of 80% for cutaneous malignancies with incidental PNI, compared with 54% for clinical PNI, despite aggressive treatment with RT +/- surgery and/or chemotherapy.70
A study on PNI in SCC demonstrated that the presence of additional tumor-related high-risk factors was associated with poorer outcomes, and concluded that these patients should also be considered for adjuvant RT.71 Factors identified included poor differentiation, tumor diameter ≥2 cm, and invasion beyond subcutaneous adipose tissue. Significantly, patients with involvement of large nerves (≥0.1 mm) were also found to be more likely to have such concomitant adverse features.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree