64.1
Introduction
Low-trauma fractures are considered as the clinical significance of bone fragility, classically named osteoporosis. They are defined as fractures resulting from a fall from standing height or less, or from a trauma from which a fracture would not be expected in a healthy individual. They are associated with low bone mineral density (BMD) and are one of the main predictors of subsequent fractures, especially in the 2 years following the index fracture (imminent fracture risk) . The most common fractures associated with osteoporosis, called “major osteoporotic fractures” (MOF) occur at the hip, spine, wrist, and proximal humerus. On the contrary, high-trauma fractures, resulting from motor vehicle crashes and falls from greater than standing height, may not systematically reveal bone fragility, since the degree of trauma applied on the bone is supposed to be greater than the failure load of a “healthy” strong bone. Part of high-trauma nonspine fractures may however be associated with low BMD and increased risk of subsequent fractures in older adults .
Low-trauma fractures represent a major public health concern, more common than stroke, myocardial infarction, and breast cancer combined in postmenopausal women . It is estimated that a 50-year-old white woman has a 15%–20% lifetime risk of hip fracture and a 50% risk of any osteoporotic fracture. About one in five men aged 50 years and over will experience a low-trauma fracture .
Low-trauma fractures significantly impair patients’ health-related quality of life, and generate at the societal level high direct and indirect health-care costs. Patients with bone fragility often experience multiple recurrent fractures, leading subsequently to loss of mobility, of independence, higher rates of institutionalization, and higher mortality rates . Fragility fractures are also associated with an increased mortality risk . In this context, identification of subjects at high risk of fracture remains challenging for the clinician, especially because interventions targeting modifiable risk factors of bone fragility can be implemented, and because several drugs are available to increase bone strength and therefore decrease the risk of fracture . The value of noninvasive imaging techniques to predict fractures will be discussed in this chapter.
64.2
Dual-energy X-ray absorptiometry
64.2.1
The definitions of osteoporosis
Osteoporosis is characterized by a decrease in BMD and alterations of bone geometry and microarchitecture, leading to bone fragility and fractures (clinical definition). An operational definition of osteoporosis, proposed by the World Health Organization, is used in clinical practice to define osteoporotic status of patients, which correspond to an areal BMD (aBMD), assessed by dual-energy X-ray absorptiometry (DXA), that lies 2.5 standard deviations (SD) or more below the average value for young healthy women (T-score ≤−2.5 SD) ( Table 64.1 ). BMD is classically assessed at the lumbar spine and the hip (central DXA), with the femoral neck (FN) as the reference site . The use of peripheral DXA (distal radius) in clinical practice has been poorly defined and is currently not recommended, except in the case of impossibility to examine central bones .
| Status | Bone mineral density |
|---|---|
| Normal | T-Score of −1 or above |
| Osteopenia | T-Score lower than −1 and greater than −2.5 |
| Osteoporosis | T-Score of −2.5 or lower |
| Severe osteoporosis | T-Score of −2.5 or lower, and presence of at least one fragility fracture |
64.2.2
Central dual-energy X-ray absorptiometry and fracture prediction
Although it is well established that a T-score ≤−2.5 SD is associated with a substantial increase in fracture risk, the limits of the operational definition of osteoporosis have rapidly been highlighted. A high proportion of fragility fractures occurs in patients with osteopenia on central (hip or spine) DXA. This has been illustrated in elderly women in the Rotterdam Study , or in patients admitted with a fragility fracture in Fracture Liaison Services: for instance in Geneva, 55% of subjects seen in the context of a low-trauma fracture have no osteoporosis on DXA (44% with osteopenia and 11% with normal BMD). The high proportion of nonosteoporotic patients is observed for the majority of fracture sites, including sites of classical MOF (hip, vertebra, humerus, and wrist).
64.2.3
Combining bone mineral density and clinical risk factors of fracture
Identification of subjects at high risk of fracture solely based on central DXA remains challenging. Multiple tools, complementary to DXA, have been developed to predict fracture risk, based essentially on clinical risk factors of fracture±BMD. The Fracture Risk Assessment Tool (FRAX, available at https://www.sheffield.ac.uk/FRAX/?lang=en ) estimates the 10-year absolute fracture risk by incorporating several clinical risk factors of fracture and FN aBMD . Risk factors included in the models are age, sex, weight, height, prior fractures, history of hip fracture in the patient’s mother or father, alcohol and tobacco consumption, glucocorticoids use, rheumatoid arthritis, and secondary causes of osteoporosis [type I diabetes, osteogenesis imperfecta in adults, untreated long-standing hyperthyroidism, hypogonadism or premature menopause (<45 years), chronic malnutrition, or malabsorption and chronic liver disease]. However, a large proportion of low-trauma fractures occurs in patients with relatively few clinical risk factors, hence modest fracture probability score by FRAX.
64.3
Contribution of bone microstructure to fracture risk
64.3.1
Microstructural determinants of bone fragility
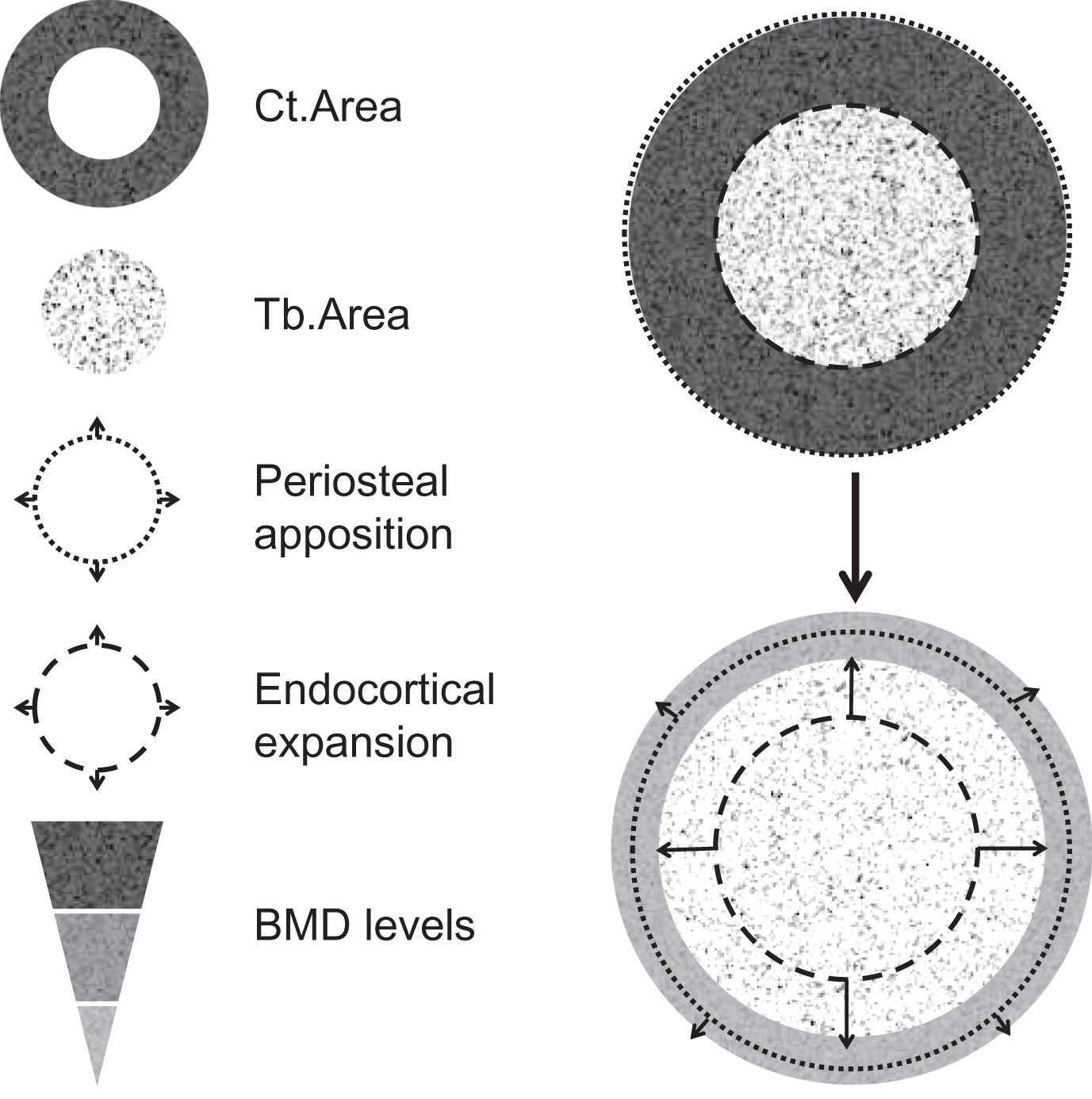
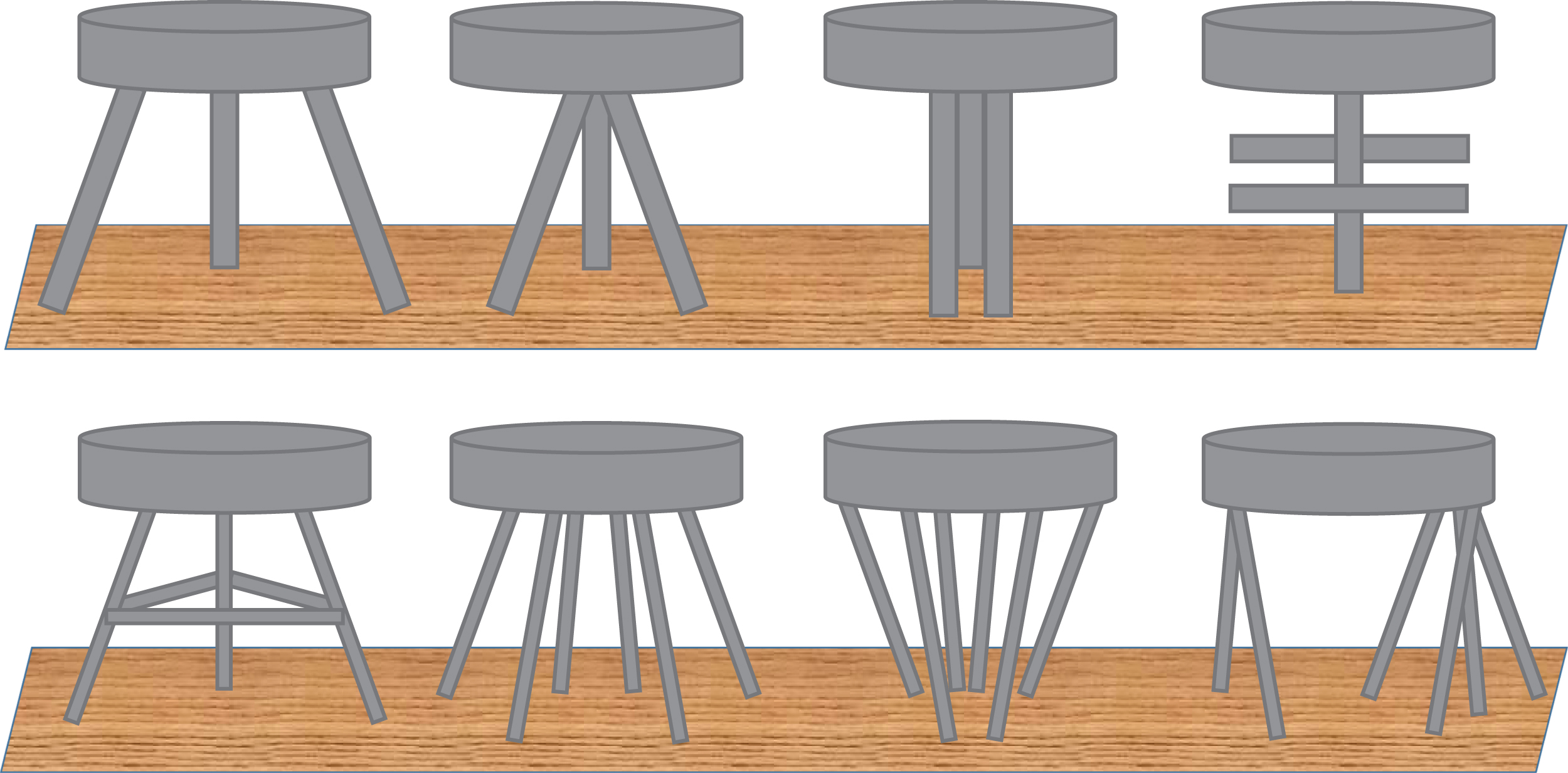
Bone strength results on the combination of bone mass, geometry, and microstructure. One of the reasons of the poor performance of DXA or FRAX to identify patients who will sustain a fracture is that alterations of bone microarchitecture (as part of the clinical definition of osteoporosis) are not captured by these tools, although they contribute to bone fragility in addition to bone mass (BMD). With the DXA-based operational definition of osteoporosis based solely on bone mass, the component of bone microarchitecture of the clinical definition is missed. It is however well established that age-related bone fragility is associated with alterations in “bone quality,” including trabecular (Tb) bone loss, cortical (Ct) thinning, and porosity, in addition to the decline in aBMD . Age-related changes in bone ( Fig. 64.1 ) include periosteal apposition with outward Ct expansion, which may have beneficial effects on bone strength, but which are not sufficient to compensate the increase of endocortical resorption, of Ct porosity, and the large decrease in Tb and Ct BMD, which account for the most important cause of increased skeletal fragility in the elderly . Fig. 64.2 illustrates that for a given mass of material, the microarchitecture is a crucial determinant of the mechanical strength of the structure. The same concept can be applied regarding the contribution of bone microstructure to bone fragility and fracture risk, independently of BMD. Based on the observation that a large proportion of low-trauma fractures occurs in patients with osteopenia or relatively few clinical risk factors, hence modest fracture probability score by FRAX, alterations of bone quality, especially bone microarchitecture, may contribute to bone fragility in addition to aBMD and may not be captured by the tools used in clinical practice to assess fracture risk (FRAX±BMD).
64.3.2
Assessment of bone microstructure and strength
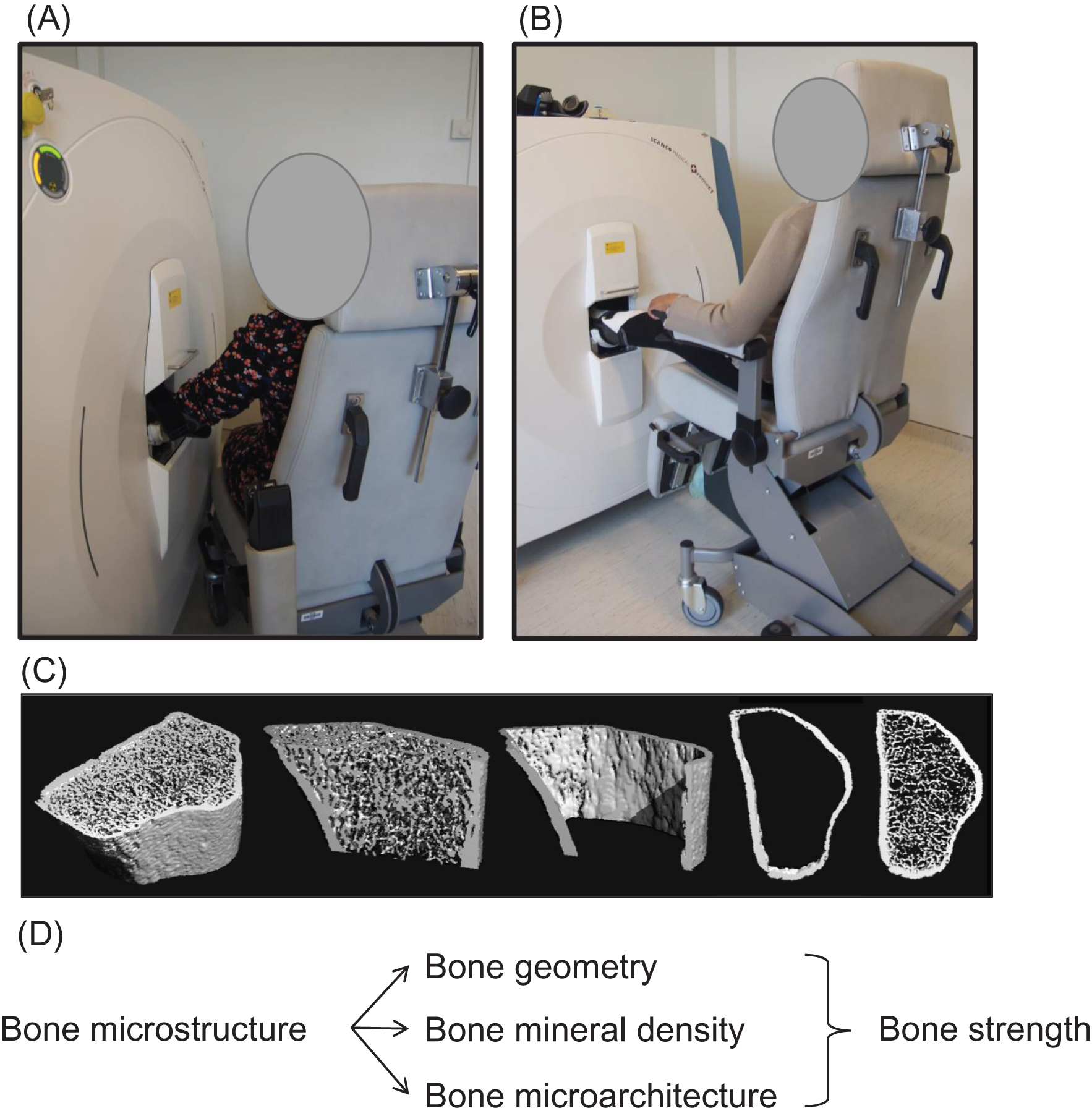
Bone microstructure can be assessed noninvasively in vivo at the distal radius and tibia, using a high-resolution (60/82 μm) peripheral quantitative computed tomography (HR-pQCT, Scanco Medical, Brüttisellen, Switzerland), suitable for in vivo studies in humans . This tool provides information on bone microstructure, including bone geometry, volumetric BMD (vBMD), and microarchitectural bone traits for the total bone and for two separate compartments ( Fig. 64.3 ):
- •
The Ct compartment, corresponding to compact bone at the bone surface (outer layer of bones), covered by a periosteum on its outer surface, and an endosteum on its inner surface, and particularly developed in diaphysis of long bone. Ct bone is a major determinant of bone strength and fracture risk. The increase in bone fragility with aging results from reduced periosteal bone formation and increased endocortical resorption, leading to Ct thickening, in addition to increased intracortical resorption, leading to the increase of Ct porosity .
- •
The Tb compartment, corresponding to cancellous bone, typically found in vertebrae and within the medullary cavity of the metaphysis and epiphysis of long bones. It is made of trabeculae in contact with bone marrow and hematopoietic stem cells. Accelerated and unbalance bone remodeling leads to trabeculae thinning and perforation, which contribute to the decrease of bone strength .
In addition, bone strength can be estimated by micro–finite element analysis (FEA) using finite element models of the radius and the tibia created directly from the segmented HR-pQCT images .
By providing a noninvasive method for in vivo 3D characterization of human bone, HR-pQCT considerably moved forward the field of bone imaging to better understand the microstructural deteriorations leading to bone fragility with aging and in various medical conditions. With an isotropic nominal resolution of 62/82 µm, this technology permits quantification of the geometric, microarchitectural, densitometric, and mechanical properties of Ct and Tb bone in the appendicular skeleton (distal radius and distal tibia) and has been used as a gold standard for noninvasive bone microstructure assessment.
64.3.3
Bone microstructure and fracture prediction
Microstructural bone traits measured by HR-pQCT allow the discrimination between osteopenic women with and without prior fragility fractures . Microstructure alterations are aBMD-independent determinants of fracture risk, which contribute to bone mechanical properties . These data suggest that microtructure alterations not captured by aBMD measured by DXA may characterize bone fragility in nonosteoporotic subjects with fractures. In a large multicenter study, including 470 fractured postmenopausal white women among 1379 women, significant and consistent deficits in vBMD and microarchitecture independent of total hip aBMD were found at both the distal radius and tibia in women with fracture compared to those who never had a fracture . Alterations of bone microstructure are also identified in subjects with ankle fractures that are classically not considered as an osteoporotic fracture .
Several prospective cohort studies have shown that HR-pQCT can predict incident fractures in men and women , independently of DXA BMD and, in some cases, of FRAX, even with inclusion of FN aBMD ( Table 64.2 ). These studies differed regarding the age and sex of the population, the duration of follow-up and their power was limited by low numbers of incident fractures ( n =22–135). Some of them suggested that the main independent contributors to fracture risk were the Tb parameters , while others found these were the Ct ones . Both the Tb and Ct parameters predict incident fractures, independently of each other . As a result of these data, the parameters, including both Tb and Ct compartments, Tt vBMD and FEA-derived strength parameters (failure load) were identified as strong independent predictors of fractures. The addition of peripheral BMD to FN BMD improves the areas under the curve for fracture prediction. Peripheral BMD and microarchitecture also predicted incident fractures in subgroups of women, which would not have been qualified at high risk of fracture with the classical tools, that is, nonosteoporotic women on central DXA or women with FRAX score below the intervention threshold for age. Last, the magnitude of the associations between radius BMD and incident fracture was also higher in women with multiple fractures and imminent fractures. Taken together, these data suggested that the prediction of low-trauma fractures in postmenopausal women can be improved with the assessment of peripheral bone microstructure and strength assessed by HR-pQCT, beyond the tools currently used in clinical practice to assess fracture risk and guide interventions with antiosteoporotic drugs (mainly FN aBMD and FRAX).
| Study (reference) | Sex, age (years) | Incident fractures | Associations with fractures | Associations with fractures in adjusted models | ||||
|---|---|---|---|---|---|---|---|---|
| n Subjects, follow-up (years) | Tt vBMD | Tb microstructure | Ct microstructure | Strength (failure load) | Adjustments | Results after adjustment | ||
| MrOS Sweden n =456 | M, 80.2±3.5 | 71, 5.3±2.0 | Not reported | Yes (Tib, Rad not assessed) | Yes (Tib, Rad not assessed) | Yes (Tib, Rad not assessed) | Age, BMI, FN-aBMD FRAX with FN-aBMD |
|
| OFELY n =589 | F, 68±9 | 135, median 9.4 IQR 1.0 | Yes (Rad+Tib) | Yes (Rad+Tib) | Only Ct Ar | Yes (Rad+Tib) | Age, current smoking, falls in the past year, prior Fx, osteoporosis-related drug use, and total hip BMD | Only Tt vBMD, strength and radius Tb parameters remained significant |
| GERICO n =740 | F, 65±1.4 | 68, 5.0 ± 1.8 | Yes (Rad+Tib) | Yes (Rad>Tib) | Yes (Rad+Tib) | Yes (Rad+Tib) | FN aBMD; FRAX–BMD; FRAX–BMD+TBS; UD radius aBM |
|
| STRAMBO n =825 | M, 60–87 | 105, 8.0 (IQR 6.0, 8.0) | Yes (Rad+Tib) | Yes (Rad+Tib) | Yes (Rad+Tib) | Yes (Rad+Tib) | Age, BMI, prior falls, and fractures; distal radius or total hip aBMD |
|
| Osteoporotic Fractures in Men (MrOS) study n =1794 | M, mean 84.4, range 77–101 | 108, 1.7 (IQR 1.4, 2.3) | Yes (Rad+Tib) | Yes (Rad+Tib) | Yes (Rad+Tib) | Yes (Rad+Tib) | Clinical site, limb length, major osteoporotic fracture risk (FRAX with BMD), total hip BMD, age, race, falls, prevalent fracture after age 50 years |
|
| CaMos n =149 | F, 68.2±7.0 (no Fr) 69.6±6.1 (Fr) | 22, 5±0.5 | Yes (Rad+Tib | Yes (Rad>Tib) | Only Ct area and thickness, not Ct BMD, and only at the tibia | Yes (Tib) | No | The rate of bone loss was not different between fracture groups |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree