For more than 50 years, vitamin K antagonists (VKAs) have been the standard of care for treatment of atrial fibrillation (AF). However, the numerous limitations of VKAs have led to the development of non-VKA oral anticoagulants (NOACs). There are 4 NOACs currently approved for prevention of thromboembolism in patients with nonvalvular AF. This article provides an overview of AF, summarizes basic properties of NOACs, and reviews the landmark trials. Current data on use of NOACs in special populations and specific clinical scenarios are also presented. Lastly, recommendations from experts on controversial topics of bleeding management and reversal are described.
Key points
- •
Non–vitamin K antagonist oral anticoagulants (NOACs) are effective in preventing stroke and systemic embolic events in patients with atrial fibrillation and have a superior safety profile compared to warfarin.
- •
Analyses in special populations allow for a versatile use of NOACs.
- •
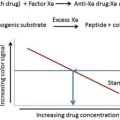
Reversal agents for Factor Xa inhibitors are in the final stages of development; a reversal agent for dabigatran is now available.
Introduction
Atrial Fibrillation: Definition and Epidemiology
Atrial fibrillation (AF) is the most common cardiac arrhythmia with an incidence of 28 per 1000 person-years in the United States. It is estimated that by 2050, the number of patients with AF will increase by 2.5 fold, affecting 6 to 12 million Americans.
The causes of AF are multifactorial, and it is a challenging disease to manage. It is associated with 5-fold increased risk of stroke, 3-fold increased risk of heart failure, and 2-fold increased risk of dementia and mortality. AF is also associated with multiple readmissions and hospitalizations as well as more than 99,000 deaths with an estimated national incremental cost of $26 billion annually for a patient with AF versus no AF.
Based on the findings from the Central Registry of the German Competence NETwork on Atrial Fibrillation (AFNET) study, the most common symptoms in patients with AF include chest pain, palpitations, dyspnea, dizziness, and fatigue. AF can also lead to tachycardia-induced cardiomyopathy. Although AF remains a significant risk factor for stroke, other underlying causes of stroke, such as local plaque rupture, atherothrombotic emboli from intracranial and extracranial arteries, carotid dissection, and presence of inflammation and coagulopathies, should be considered. This article focuses on stroke prevention using oral anticoagulants in AF.
AF is classified based on the duration of episodes. The pivotal trials that compared Non-Vitamin K antagonist oral anticoagulants (NOACs) with warfarin enrolled patients with nonvalvular AF (NVAF). However, the definition of NVAF has varied between the trials; there is a lack of consensus on the definition of valvular and nonvalvular among various guidelines. Nonetheless, it is well established that the risk of thromboembolism is particularly high in AF associated with moderate to severe mitral stenosis and mechanical valves. Furthermore, it remains unknown whether the pathogenesis of thrombogenesis is different in these conditions versus other forms of AF; thus, it has been proposed to keep “mechanical and rheumatic mitral valvular AF” separate from other types of AF.
AF occurs when there is a change in the atrial architecture promoting propagation and maintenance of abnormal electrical activity ( Fig. 1 ). Conditions such as hypertension, coronary artery disease, and various cardiomyopathies on a cellular level are characterized by fibrosis, inflammation, and hypertrophy, which predispose to AF. AF itself leads to further changes in the left atrium, endothelial damage, myocytic hypertrophy, necrosis, and mononuclear cell infiltrates, all of which predispose a patient to a hypercoagulable state. Multiple genes have also been identified that predispose to AF. Most of the mutations in AF are gain-of-function mutations in potassium channels that lead to increased probability of channel opening, which in turn increase atrial action potential duration and atrial refractory period. Loss-of-function mutations in potassium channels lead to early after-depolarizations and AF. Currently genome-wide association studies have identified numerous genes that encode gap junctions, transcription factors involved in sodium homeostasis, and cardiac transcription factors that play a role in AF. It is hypothesized that ectopic atrial foci composed of atrial myocardial fibers around pulmonary veins are particularly arrhythmogenic. Pulmonary vein myocytes have been shown to reduce upstroke velocity and decrease resting membrane potential associated with delayed rectified current and decreased action potential duration. In addition to pulmonary veins, other sites, such as the posterior left atrium, ligament of Marshall, coronary sinus, venae cavae, septum, and appendages, serve as foci for AF.
Warfarin Efficacy, Safety, and Limitations
Vitamin K antagonists (VKAs) have been the cornerstone of therapy for stroke prevention in AF. The anticoagulant effect of warfarin is measured by the international normalized ratio (INR). Based on stroke prevention trials in AF and cohort studies, the recommended INR range for prevention of stroke in patients with NVAF has been established to be 2.0 to 3.0. A decrease in INR from 2.0 to 1.7 was previously shown to double the risk of ischemic stroke, and a further decrease to 1.4 further doubled the risk yet again. An INR greater than 4 is associated with increased risk of subdural hemorrhage.
The proportion of time spent in the INR range of 2.0 to 3.0, or time in therapeutic range (TTR), has been validated as a marker to predict outcomes of anticoagulation. Based on the post hoc analysis of the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE W) trial, a target threshold of TTR of at least 58% to 65% was necessary to demonstrate a benefit of warfarin over dual antiplatelet therapy in stroke prevention in AF.
Nonetheless, there are numerous limitations to TTR. First, as TTR is a measure of anticoagulation over time, transient fluctuations in INR are not reflected by TTR; thus, risk of stroke or hemorrhage during these periods is difficult to assess. Second, TTR is valid when INR is measured over consecutive periods of time; when there are gaps of more than 56 days, TTR is no longer accurate.
Because warfarin has a narrow therapeutic range and there is great variation in daily dose requirements based on patients’ characteristics, there is a risk of overanticoagulation and underanticoagulation. Given the difficulty in predicting the level of anticoagulation when initiating therapy with warfarin, genotype-guided dosing has also been studied. Polymorphisms in genes CYP2C9 and VKORC1, along with body surface area and age, have been shown to account for more than 50% variability in dosing. In the 455-subject prospective multicenter, randomized controlled trial, pharmacogenetic-based dosing versus control was associated with higher mean percentage of TTR (67.4% vs 60.3% adjusted difference, 7.0% points; 95% confidence interval [CI], 3.3–10.6) with fewer incidences of excessive anticoagulation and decreased time needed to reach therapeutic INR (21 vs 29 day [ P <.001]). Furthermore, a subgroup analysis of the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE-AF TIMI 48) trial showed that patients with CYP2C9 and VKORC1genotypes who corresponded to US Food and Drug Administration (FDA) categories of sensitive and highly sensitive responders to warfarin were more likely to be overanticoagulated and had higher rates of bleeding in the first 90 days of initiating treatment. Edoxaban, compared with warfarin, was associated with significantly less bleeding events, including fatal, life-threatening, intracranial, and major bleeding. Although these results are promising, genotyping before warfarin prescription is not recommended given the lack of sufficient randomized controlled trial data, relatively brief window of utility, and uncertain cost-benefit.
Furthermore, other patient-specific factors that influence INR include nonwhite race, female sex, poverty, greater distance from care, active cancer, frequent hospitalizations, chronic liver disease, substance abuse, dementia, and major depression.
Although there is more than 60 years of experience with warfarin as the only oral anticoagulant option, there are numerous limitations associated with its use. Warfarin has slow onset and offset of action, which is associated with long hospital stays and long periods of time to reach homeostasis. Furthermore, there are genetic variations in warfarin metabolism as well as numerous food and drug interactions, which make it difficult to achieve therapeutic levels of anticoagulation.
Non–Vitamin K Antagonist Oral Anticoagulants (NOACs)
The many disadvantages of warfarin led to the development of NOACs. The predictable anticoagulant activity, short half-life, and minimal drug-drug interactions make NOACs a favorable alternative to warfarin. As is discussed further, the pivotal trials that compared NOACs with warfarin proved the comparable efficacy of NOACs in reducing the risks of stroke and thromboembolism and superior safety profile in reducing the risk of bleeding and mortality.
There are 4 NOACs currently approved in the United States, Europe, and Asia. They are dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran was approved by the US FDA for prevention of stroke in patients with NVAF in 2010, followed by rivaroxaban (2011), apixaban (2012), and most recently edoxaban (2015) ( Table 3 ).
Dabigatran
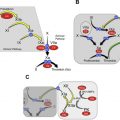
Dabigatran (Pradaxa) is a direct thrombin inhibitor, blocking the free- and clot-bound thrombin and thrombin-induced platelet aggregation ( Fig. 2 ). Its bioavailability is 3% to 7% and reaches peak plasma concentration in 1 to 2 hours after ingestion. The half-life of dabigatran ranges from 12 to 17 hours in patients with normal and mildly impaired renal function. However, because 80% is cleared renally, the half-life is longer in patients with severe renal insufficiency ( Table 1 ).
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|
| Trade name | Pradaxa | Xarelto | Eliquis | Savaysa (United States) Lixiana (other countries) |
| Target | Thrombin | Factor Xa | Factor Xa | Factor Xa |
| Half-life (h) | 12–17 | 5–9 11–13 (in elderly) | 12 | 10–14 |
| Peak plasma concentration (h) | 1 | 2–4 | 3–4 | 1–2 |
| Renal clearance (%) | 80 | 33 | 27 | 50 |
| Protein binding (%) | 35 | >90 | 87 | 55 |
Based on a large, randomized, open-label trial, Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY), 2 doses of dabigatran were approved for stroke prevention in patients with NVAF: 150 milligram (mg) and 75 mg; both are dosed orally twice daily. Although the 150-mg and 110-mg doses of dabigatran were studied in RE-LY, only the 150-mg dose was approved by the FDA. Both doses were noninferior to warfarin in preventing stroke and systemic embolic events (SEE); however, the 150-mg dose was superior to warfarin in terms of efficacy, and the 110-mg dose was associated with significantly fewer bleeding events than warfarin. The 150-mg dose reduced the risk of stroke and SEE more than the 110-mg dose but also caused more bleeding. Subgroup analyses in more vulnerable patients (patients >75 years of age and those with moderate renal impairment [creatinine clearance (CrCL) 30–50 mL/min]) further confirmed these findings. Given that the irreversible sequelae of stroke is more clinically significant than nonfatal bleeding and the risk of undertreating with a lower dose would be associated with more strokes, the higher dose of dabigatran was favored.
In the United States, the FDA approved the use of 75 mg dose based on pharmacokinetic and pharmacodynamic data in patients with a calculated CrCl of 15 to 30 mL/min. The 75-mg dose should also be considered in patients with CrCl 30 to 50 mL/min who are taking a concomitant P-glycoprotein inhibitor. Outside the United States, the approved doses are 150 mg and 110 mg, with the lower dose preferred in patients at increased risk of bleeding, such as elderly patients and those with moderate renal dysfunction ( Table 2 ).
| NOAC | Dabigatran | Rivaroxaban | Apixaban | Edoxaban |
|---|---|---|---|---|
| Trial name | RE-LY | ROCKET-AF | ARISTOTLE | ENGAGE AF-TIMI 48 |
| US FDA–approved dosing | For CrCl >30 mL/min: 150 mg BID For CrCl 15–30 mL/min: 75 mg BID | For CrCl >50 mL/min: 20 mg Qd with evening meal For CrCl 15–50 mL/min: 15 mg Qd with the evening meal | 5 mg BID In patients with at least 2 of the following: age ≥80 y, body weight ≤60 kg, or creatinine ≥1.5 mg/dL: 2.5 mg BID | For CrCl >50 to ≤95 mL/min: 60 mg Qd For CrCl 15–50 mL/min: reduce dose to 30 mg Qd For CrCl >95 mL/min: avoid use |
| Drug interactions | Avoid concomitant use with P-gp inducers For CrCl 30–50 mL/min and concomitant P-gp inhibitor a reduce dose to 75 mg BID For CrCl 15–30 mL/min avoid concomitant use with P-gp inhibitors | Avoid concomitant use with combined P-gp and strong CYP3A4 inhibitors b or moderate CYP3A4 inhibitor c Avoid concomitant use of combined P-gp and strong CYP3A4 inducers d For patients taking 2.5 mg BID dose, avoid concomitant use with strong dual inhibitors of CYP3A4 and P-gp | Decrease dose by 50% if concomitant use with strong dual inhibitors of CYP3A4 and P-gp e | Avoid use with rifampin |
a Dronedarone or systemic ketoconazole.
b Ketoconazole, ritonavir, clarithromycin, and erythromycin.
d Carbamazepine, phenytoin, rifampin, St. John’s wort.
In RE-LY, 18,113 patients with NVAF were randomized to receive dabigatran versus warfarin. The rates of stroke and SEE in the group that received the 150-mg dose of dabigatran twice daily were 1.11%/y versus 1.69%/y in the warfarin group (relative risk, 0.66; 95% CI, 0.53–0.82; P <.001 for superiority). The rates of major bleeding were 3.11%/y and 3.36%/y, respectively ( P = .31). Additional important secondary findings from the RE-LY trial are summarized in Table 3 .
| Trial | RE-LY | ROCKET-AF | ARISTOTLE | ENGAGE-AF TIMI 48 | ||
|---|---|---|---|---|---|---|
| NOAC | Dabigatran 110 mg vs VKA | Dabigatran 150 mg vs VKA | Rivaroxaban vs VKA | Apixaban vs VKA | Edoxaban 60/30 mg vs VKA | Edoxaban 30/15 mg vs VKA |
| Primary end point (stroke, SEE), HR (CI), P value for non-inferiority | ||||||
| 0.91 (0.74–1.11) P <.001 | 0.66 (0.53–0.82) P <.001 | 0.88 (0.75–1.03) b P <.001 | 0.79 (0.66–0.95) P <.001 | 0.79 (0.63–0.99) a P <.001 | 1.07 (0.87–1.31) P = .005 | |
| Principal safety outcome, HR (CI), P value | ||||||
| 0.8 (0.69–0.93) P = .003 | 0.93 (0.81–1.07) P = .31 | 1.03 (0.96–1.11) P = .44 | 0.69 (0.60–0.80) P <.001 | 0.8 (0.71–0.91) P <.001 | 0.47 (0.41–0.55) P <.001 | |
| Death any cause, HR (CI), P value | ||||||
| 0.91 (0.8–1.01) P = .13 | 0.88 (0.77–1.00) P = .051 | 0.71 (0.49–1.03) P = .075 | 0.89 (0.80–0.998) P = .047 | 0.92 (0.83–1.01) P = .08 | 0.87 (0.79–0.96) P = .006 | |
| GI bleed, HR (CI), P value | ||||||
| 1.1 (0.86–1.41) P = .43 | 1.5 (1.19–1.89) P <.001 | 1.34; P <.0001 | 0.89 (0.7–1.15) P = .37 | 1.23 (1.02–1.50) P = .03 | 0.67 (0.53–0.83) P <.001 | |
| Myocardial infarction HR (CI), P value | ||||||
| 1.35 (0.98–1.87) P = .07 | 1.38 (1.00–1.91) P = .048 | 0.81 (0.63–1.06) P = .121 | 0.88 (0.66–1.17) P = .37 | 0.94 (0.74–1.19) P = .6 | 1.19 (0.95–1.49) P = .13 | |
a Modified intention to treat population.
The Long-term Multicenter Extension of Dabigatran Treatment in Patients with Atrial Fibrillation (RELY-ABLE) registry enrolled patients who had completed participation in the RE-LY clinical trial on the study drug and then followed these patients on extended therapy for an additional 2.3 years (median). During this continued treatment period, there were similar rates of stroke and SEE in the 150-mg and 110-mg doses of dabigatran with higher rates of major bleeding with the 150-mg dose. The rates of hemorrhagic stroke and intracranial bleeding remained low in the dabigatran group, consistent with findings in RE-LY. Based on recent postmarketing surveillance data, which included 134,414 patients and 37,587 person-years of follow-up, the rates of stroke and SEE were similar among patients taking dabigatran versus warfarin with significantly fewer rates of intracranial hemorrhage (ICH). The rates of major bleeding were higher in the dabigatran group but did not reach significance.
Rivaroxaban
Rivaroxaban (Xarelto) is a direct factor Xa (FXa) and prothrombinase-bound FXa inhibitor ( Fig. 3 ). Its bioavailability is dose dependent; for the 15-mg and-20 mg doses, bioavailability increases with concomitant food intake. Rivaroxaban reaches peak plasma concentration in 2 to 4 hours. Approximately 66% of a rivaroxaban dose is eliminated via kidneys; renal metabolism accounts for 33% of its clearance, and the other 33% is excreted in urine unchanged ( Table 1 ).
There are 2 doses of rivaroxaban approved in the United States for stroke prevention in patients with NVAF: 15 mg and 20 mg. The recommended dose is 20 mg administered orally once daily with meals for CrCl greater than 50 mL/min with the dose reduced to 15 mg once daily for CrCl 15 to 50 mL/min. Concomitant use of rivaroxaban and strong permeability glycoprotein (P-gp) inhibitors/inducers and CYP3A4 inhibitors/inducers should be avoided ( Table 2 ).
Based on a large, double-blind trial, Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF), rivaroxaban was approved for stroke prevention in patients with NVAF. In the ROCKET AF trial, 14,264 patients were randomized to receive rivaroxaban versus warfarin. The rates of stroke or SEE were 1.7%/y versus 2.2%/y in the experimental and control groups, respectively (hazard ratio [HR], 0.79; 95% CI, 0.66–0.96; P <.001 for noninferiority). Furthermore, the rates of major and nonmajor clinically relevant bleeding were similar across patients receiving rivaroxaban and warfarin (14.9%/y and 14.5%/y, respectively; HR, 1.03; 95% CI, 0.96–1.11; P = .44). Key secondary findings from ROCKET AF are summarized in Table 3 .
Apixaban
Apixaban (Eliquis) is a free- and clot-bound FXa and prothrombinase-complex inhibitor (see Fig. 3 ). Its bioavailability is 50% and is not affected by concomitant food intake. It reaches peak plasma concentration in 3 to 4 hours and is eliminated in urine and feces, with urine clearance accounting for 27% of total clearance ( Table 1 ).
There are 2 doses of apixaban available in the United States: 2.5 mg and 5.0 mg dosed orally twice daily. For stroke prevention in patients with NVAF, the recommended dosage is 5.0 mg administered orally twice daily with dose reduction to 2.5 mg twice daily in patients with any 2 of the following: 80 years of age or older, body weight of 60 kg or less, or serum creatinine of 1.5 mg/dL or greater. Dose reduction by 50% is recommended when coadministering a 5-mg dose of apixaban with strong inhibitors of cytochrome P450 3A4 (CYP3A4) and P-gp. Concomitant use of apixaban (at any dose) with strong dual inhibitors of CYP3A4 and P-gp is not recommended ( Table 2 ).
Based on a large double-blind trial, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE), apixaban was approved for stroke prevention in patients with NVAF. In ARISTOTLE, 18, 201 patients with NVAF were randomized to receive apixaban versus warfarin. The rates of stroke or SEE in apixaban and warfarin groups were 1.27%/y and 1.60%/y, respectively (HR, 0.79; 95% CI, 0.66–0.95; P = .01 for superiority). Patients who received apixaban also had lower rates of bleeding compared with warfarin (2.13%/y and 3.09%/y, respectively; HR, 0.69; 95% CI, 0.60–0.80; P <.001). Additional key findings from ARISTOTLE are summarized in Table 3 .
Apixaban was compared with aspirin in patients who were not willing or able to take a VKA in a double-blind randomized trial known as Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES). The trial enrolled 5599 patients with AF and increased risk of stroke, who were treated with either apixaban 5 mg twice daily versus aspirin 81 to 324 mg once daily. The trial was terminated early as clear benefit of apixaban over aspirin was seen in preventing stroke and SEE with similar rates of bleeding.
Edoxaban
Edoxaban (Savaysa in the United States, Lixiana outside the United States) is an FXa inhibitor with greater than 10,000-fold increased affinity to FXa than thrombin (see Fig. 3 ). Its bioavailability is 62%, and it reaches peak plasma concentration in 1 to 2 hours. It has dual renal (50%) and extrarenal clearance ( Table 1 ).
There are 3 available doses approved in the United States: 15 mg, 30 mg, and 60 mg dosed orally once daily. For stroke prevention in patients with NVAF, the recommended dosage is 60 mg once daily in patients with CrCL greater than 50 to 95 mL/min or less. Dose reduction is recommended in patients with CrCl 15 to 50 mL/min, whereas edoxaban is not recommended in those with CrCl greater than 95 mL/min. The US FDA recommends no dose reduction with concomitant P-gp inhibitor use, but coadministration with potent P-gp inducers should be avoided. Outside the United States, most regulatory authorities recommend a 50% dose reduction when concomitant strong P-gp inhibitors are used (eg, dronedarone, verapamil, quinidine), and for body weight ≤ 60 kg, with no limitation for use for CrCL >95 mL/min. When transitioning from 60 mg edoxaban to VKA, the 30-mg edoxaban dose is started concomitantly with warfarin until therapeutic INR is reached. When transitioning from 30 mg edoxaban to VKA, the 15-mg edoxaban dose is used instead ( Table 2 ).
Based on the largest and longest randomized trial of a NOAC, the ENGAGE AF-TIMI 48 trial, edoxaban was approved for stroke prevention in patients with NVAF. In the ENGAGE AF-TIMI 48 trial 21,105 patients were randomized to receive a higher-dose edoxaban regimen (60 mg with dose reduction to 30 mg), lower-dose edoxaban regimen (30 mg with dose reduction to 15 mg), or warfarin in a double-blind, double-dummy fashion. The rates of stroke and SEE were 1.18%/y and 1.61%/y in the higher- and lower-dose groups of edoxaban, compared with warfarin 1.50%/y (HR, 0.79; 97.5% CI, 0.63–0.99; P <.001 for noninferiority and HR, 1.07; 97.5% CI, 0.87–1.31; P = .005 for noninferiority, respectively). The rates of major bleeding were 2.75%/y and 1.61%/y in the higher- and lower-dose groups of edoxaban, compared with warfarin 3.43%/y (HR, 0.80; 95% CI, 0.71–0.91; P <.001 for noninferiority and HR, 0.47; 95% CI, 0.41–0.55; P <.001 for noninferiority, respectively). Other findings from ENGAGE AF-TIMI 48 are summarized in Table 3 .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree