Fig. 4.1
Incidence of NHL by age, United States SEER, 2000–2011
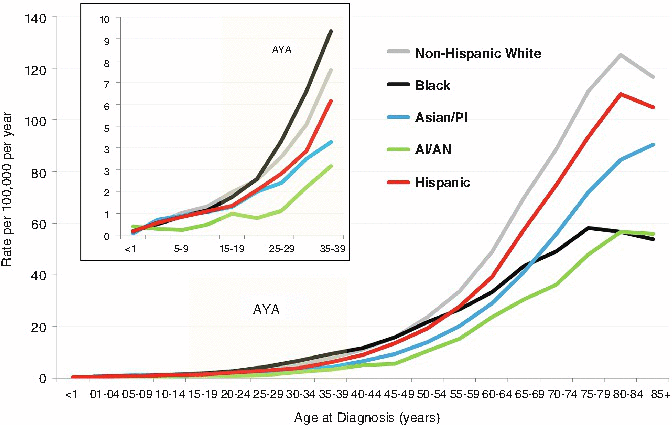
Fig. 4.2
Incidence of NHL by age and race/ethnicity; United States SEER, 2000–2011
Table 4.1
Incidence of non-Hodgkin lymphoma in persons younger than 30 years of age, United States, 1975–2000
Age at diagnosis (years) | <5 | 5–9 | 10–14 | 15–19 | 20–24 | 25–29 |
|---|---|---|---|---|---|---|
United States population, year 2000 census (in millions) | 19,176 | 20,550 | 20,528 | 20,220 | 18,964 | 19,381 |
Non-Hodgkin lymphoma, iCCC ii(b) | ||||||
Average incidence per million, 1975–2000, SEER | 3.4 | 5.4 | 7.1 | 11.7 | 16.5 | 27.3 |
Average annual % change in incidence, 1975–2000, SEER | na | 0.2 % | 2.2 % | 2.3 % | 3.6 % | 6.2 % |
Estimated incidence per million, year 2000, United States | 2.8 | 5.5 | 8.8 | 14.3 | 21.8 | 39.3 |
Estimated number of persons diagnosed, year 2000, United States | 66 | 110 | 147 | 290 | 413 | 762 |
Burkitt and other non-Hodgkin lymphomas, iCCC ii(c), ii(d), and ii(e) | ||||||
Average incidence per million, 1975–2000, SEER | 2.8 | 3.7 | 3.9 | 3.1 | 3.6 | 7.5 |
Average annual % change in incidence, 1975–2000, SEER | na | −1.0 % | −0.7 % | 1.6 % | 9.8 % | 18.5 % |
Estimated incidence per million, year 2000, United States | 1.9 | 3.2 | 3.6 | 3.5 | 5.6 | 12.5 |
Estimated number of persons diagnosed, year 2000, United States | 54 | 76 | 81 | 72 | 108 | 243 |
4.2.2 Incidence of Histologic Types
The predominant NHL in AYA were DLBCL and anaplastic large cell lymphoma (ALCL) which accounted for 55–70 % and 20–25 % of the NHL in AYA, respectively. Furthermore, primary mediastinal large B-cell lymphoma (PMLBCL) and NK/T-cell lymphoma had their greatest proportion in the AYA period (Fig. 4.3). The histological subtype of NHL in AYA with the greatest divergence in race/ethnicity distribution was NK/T-cell lymphoma that occurred more in Hispanics and Asian/Pacific Islanders compared to other ethnic types (Fig. 4.4).
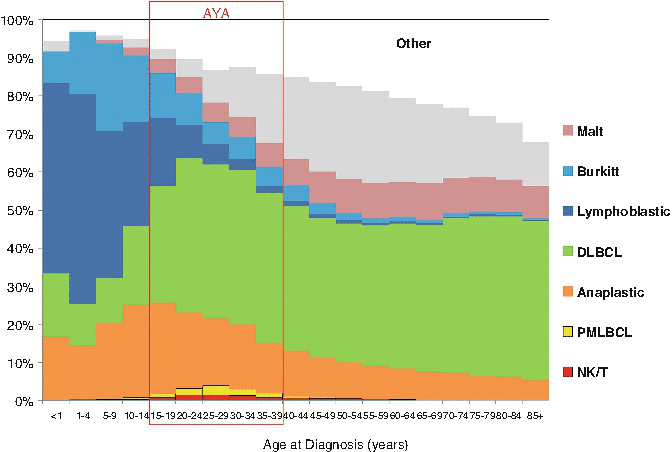
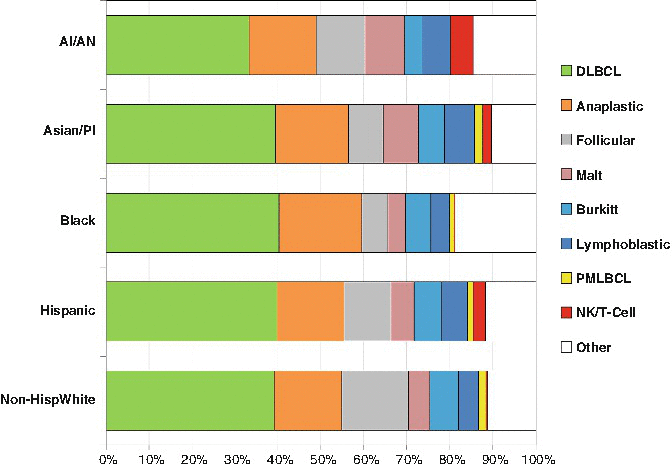

Fig. 4.3
NHL distribution of histologic types by age, United States SEER 2000–2011

Fig. 4.4
NHL distribution of histologic types by race/ethnicity, ages 15–39, United States SEER 2000–2011
The French-American-British/Lymphoma Malignancy B (FAB/LMB) 96 study, a 5-year prospective international study for the treatment of B-cell lymphoma in children and adolescents, was not a population-based registry, but interestingly some differences were observed between the three countries in terms of repartition of the two subgroups of B-cell NHL. After adjusting for age, DLBCL was more frequent in the United States than in the European countries, especially in France [2, 3].
4.3 Etiology/Risk Factors
Whatever the age, it is known that a few patients are at increased risk of developing NHL: those with congenital or acquired immunodeficiency and those receiving immunosuppressive therapy (such as after organ transplantation). The incidence is significantly higher in males than in females and is higher in whites than African American/blacks, as reviewed earlier. Specific geographical areas are also recognized for particular types of lymphoma, such as the “endemic” (African) BL. Other risk factors include Epstein-Barr virus (EBV) or Helicobacter pylori infection, tobacco, and chemical or other environmental exposures. In underdeveloped countries, there is a documented link between EBV and BL, while in the developed world, EBV is also associated with other subtypes of NHL. Secondary neoplasms are well-documented sequelae of HIV infection and account for an increase in NHL incidence, particularly in males. The HIV/acquired immune deficiency syndrome (AIDS) epidemic increased the incidence of NHL in young men 25–49 years of age. The effect of HIV/AIDS on NHL peaked in 1995 and subsided in 1999, since which the incidence of NHL has been relatively stable in both male and female AYA (Fig. 4.5). A few familial cases of lymphoid malignancies have been observed, without apparent recognized genetic abnormalities.
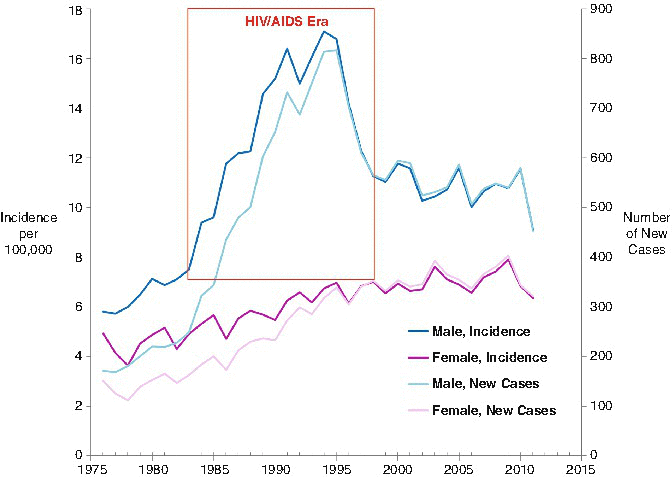

Fig. 4.5
Annual incidence of NHL by sex, ages 25–49, 1976–2011, SEER 9, United States SEER data
4.4 Histology/Cytogenetics
Classification of NHL has changed many times over the years and became more distinct with the increased understanding of lymphomagenesis and the development of new diagnostic tools (immunophenotyping, cytogenetics, molecular biology, and now gene profiling). The current World Health Organization (WHO) classification [4], preceded by the Revised American European Lymphoma (REAL) classification [5], is now widely used. Microarray technologies, by studying the expression of many genes at once, are very promising [6], but their implication for diagnosis and prognosis and their further utility in clinical practice, especially in AYA, require further investigation. The characteristics of the four categories of lymphoma most frequently encountered in AYA (BL, LL, DLBLC, and ALCL) are demonstrated in Table 4.2.
Table 4.2
Different subtypes of non-Hodgkin lymphoma in adolescents and young adults and their clinical and biologic characteristics
Burkitt | Lymphoblastic | Large B-cell | Anaplastic large cell | |
|---|---|---|---|---|
Preferential tumor site | Abdomen head and neck | Mediastinum (T cell) Bone, (sub)cutaneous (B cell) | Abdomen, thymus, bone | Node, skin |
Histology | ||||
Cell size | Medium | Medium | Large | Voluminous |
Cytoplasm nucleus nucleoli | Narrow Basophilic, with vacuoles round | Narrow, pale Convoluted or not | Cleaved or not, vesicular | Abundant and clear erythrophagocytosis irregular, clear |
Chromatin | Several nucleoli Coarse and irregular | Poorly discernable nucleoli Finely stippled | Distinct, often adherent to nucleus membrane | Voluminous |
FaB equivalent | L3 | L1, L2 | NA | NA |
Immunophenotyping | CD20+ CD79a + SIg + Ki 67 + >95 % | TdT+ B lineage T lineage CD19+, CD7+ CD 79a+, CD2+ S Ig−, CD3c+ cMu ∓ | CD20+ CD79a+ SIg ± bcl6 ± Ki 67: 60–90 % | CD30+ EMA+ ALK+ (T or “null” markers) |
Cytogenetics | t (8;14)(q24;q32) or variant t(2;8) (p11;q24) t(8;22) (q24;q11) | No specific abnormalities, sometimes involvement of T-cell antigene receptor genes (TCR) on chromosome 7(q34) or 14(q11) | Sometimes t(8;14)(q24;q32) der (3)(q27) (bcl6) | t(2;5) (p23;q35) or variant |
Result | Transcriptional deregulation of c-MYC | Transcriptional deregulation of bcl6 | NPM/ALK-fusion protein; ALK is a tyrosine kinase receptor located on 2p23 | |
4.5 Clinical Features
The clinical presentation of NHL in AYA, as in other age classes, varies and depends on the primary site of the disease, the histological subtype, and the extent of the disease. BL generally arises in the abdomen (digestive track) and in the Waldeyer ring, while LL generally arises from the thymus. Burkitt abdominal lymphoma generally presents as a large and rapidly growing abdominal mass that is often associated with ascites and other intra- or extra-abdominal involvements. Intussusception leading to the discovery of a small excisable abdominal tumor is a rare presentation that is related to BL or DLBCL. Extensive abdominal surgery should be avoided. The diagnosis can be made on surgical biopsy but also on cytological examination of a serious effusion or on percutaneous needle biopsy of the tumor.
Lymphoblastic mediastinal lymphoma leads to mediastinal compression, which may be life threatening (general anesthesia should be avoided if possible) and is often associated with a concomitant pleural effusion. Therefore, the diagnosis should be made using cytological examination of effusions or bone marrow smears. If a tumor biopsy is needed, then this should be done by percutaneous needle biopsy or by mediastinoscopy. Another lymphoma arising in the thymus is the primary mediastinal B-cell lymphoma of thymus origin, which may present with pericarditis, pulmonary nodules, and/or subdiaphragmatic involvement such as the kidney and pancreas.
Head and neck primary sites including Waldeyer’s ring and the facial bones are more often seen in BL. In the less frequent sites such as the superficial lymph nodes, bone, skin, thyroid, orbit, eyelid, kidney, and epidural space, any subtype of lymphoma can be seen, emphasizing the necessity of a good-quality sample of histology and immunophenotyping.
ALCL present with more unique features: usually nodal involvement, sometimes painful, which is characteristic of this disease; frequent skin involvement with inflammatory symptoms of the involved nodes, distant macular lesions, or general skin modification resembling ichthyosis; frequent general symptoms with widely fluctuating fever; and “wax and wane” evolution in few cases with previous episode(s) of spontaneous regression.
4.6 Initial Workup and Staging
Diagnosis can be obtained utilizing biopsy material including tumor-touch preparations but also cytological examination of effusion fluids or bone marrow smears, so surgical procedures can be avoided in diffuse BL and lymphoblastic lymphoma (LL) diseases. Also strongly recommended are the immunological and cytogenetic or molecular biology studies.
Staging classifications are different in children compared to adults. Historically, the St Jude (Murphy classification) [7] was used for children but has recently been updated to the new International Pediatric NHL Staging System (IPNHLSS) (Tables 4.3, 4.4, and 4.5) [8]. Once the diagnosis of NHL has been made, a speedy assessment of diagnosis, staging, and general evaluation must be done to commence appropriate treatment as soon as possible. This is particularly important in BL and LL, which have a great propensity to spread rapidly both regionally and systemically, especially in the bone marrow and CNS. Staging classifications are different in children, where the St Jude (also called Murphy) classification [7] is used because of the predominance of extranodal primaries, and in adults where the Ann Arbor classification, more adapted to nodal disease, is used (Table 4.6). These two different staging systems between children and adults make comparisons between pediatric and adult studies difficult, particularly in the adolescent and young adult age range. Also utilized for therapeutic classification in adults is the International Prognostic Index (IPI) based on stage, serum lactate dehydrogenase (LDH) levels, and performance status (PS) (Table 4.6).
Stage I |
A single tumor (extranodal) or single anatomical area (nodal), with the exclusion of mediastinum or abdomen |
Stage II |
A single tumor (extranodal) with regional node involvement |
Two or more nodal areas on the same side of the diaphragm |
Two single (extranodal) tumors with or without regional node involvement on the same side of the diaphragm |
A primary gastrointestinal tract tumor, usually in the ileocecal area, with or without involvement of associated mesenteric nodes onlya |
Stage III |
Two single tumors (extranodal) on opposite sides of the diaphragm |
Two or more nodal areas above and below the diaphragm |
All the primary intrathoracic tumor (mediastinal, pleural, thymic) |
All extensive primary intra-abdominal diseasea |
All paraspinal or epidural tumors, regardless of other tumor sites |
Stage IV |
Any of the above with initial involvement of the CNS and/or bone marrow (BM) involvementb |
Table 4.4
International pediatric non-Hodgkin lymphoma staging system (IPNHLSS)
Stage I |
A single tumor with the exclusion of the mediastinum and abdomen (N, nodal; EN, extra-nodal; bone (B) or skin (S), EN-B, EN-S) |
Stage II |
A single extranodal tumor with regional node involvement |
Two or more nodal areas on the same side of the diaphragm |
A primary gastrointestinal tract tumor (usually in the ileocecal area), with or without involvement of associated mesenteric nodes, that is completely resectable (if malignant ascites or extension of the tumor to adjacent organs, it should be regarded as stage III) |
Stage III |
Two or more extranodal tumors (including bone or skin: EN-B, EN-S) above and/or below the diaphragm. |
Two or more nodal areas above and below the diaphragm |
Any intrathoracic tumor (mediastinal, hilar, pulmonary, pleural, or thymic) |
Intra-abdominal and retroperitoneal disease, including liver, spleen, kidney, and/or ovary localizations, regardless of degree of resection (except a primary gastrointestinal tract tumor [usually in the ileocecal region] with or without involvement of associated mesenteric nodes, which is completely resectable) |
Any paraspinal or epidural tumor, whether or not other sites are involved |
Single bone lesion with concomitant involvement of extra-nodal and/or non-regional nodal sites |
Stage IV |
Any of the above findings with initial involvement of the central nervous system (stage IV CNS), bone marrow (stage IV BM), or both (stage IV combined) based on conventional methods; see Table 4.3 |
For each stage, type of examination and degree of BM and CNS involvement should be specified, using the abbreviations below to identify involvement; see Table 4.3 |
Table 4.5
Additional staging information
Bone marrow (BM) involvement |
Stage IV disease, due to BM involvement, is currently defined by morphological evidence of ≥5 % blasts or lymphoma cells by bone marrow aspiration. This applies to any histological subtype and will be maintained in the IPNHLSS |
However, for each stage, type and degree of BM involvement (by bone marrow aspiration) should be specified, using the abbreviations below to identify involvement: |
BMm = BM positive by morphology (specify % lymphoma cells) |
BMi = BM positive by immunophenotypic methods (immunohistochemical/flow-cytometric analysis) (specify % lymphoma cells) |
BMc = BM positive by cytogenetic/FISH analysis (specify % lymphoma cells) |
BMmol = BM positive by molecular techniques (PCR-based) (specify level of involvement) |
Same approach should be used for peripheral blood (PB) involvement (i.e., PBm; PBi, PBc, PBmol) |
Note: definition of BM involvement should be obtained from analysis of bilateral BM aspirates and BM biopsy |
Central nervous system (CNS) involvement |
CNS is considered involved in case of: |
1. Any CNS tumor mass (identified by imaging techniques, i.e., CT, MRI) |
2. In case of cranial nerve palsy that cannot be explained by extradural lesions |
3. In case of blasts morphologically identified in the CSF |
Condition that defines CNS positivity should be specified: CNS positive/mass, CNS positive/palsy, CNS positive/blasts |
CSF status: CSF positivity is based on morphological evidence of lymphoma cells |
CSF should be considered positive when any number of blasts is detected |
CSF unknown (not performed, technical difficulties, etc.) |
Similarly to BM, type of CSF involvement should be described whenever possible |
CSFm = CSF positive by morphology (specify the number of blasts/μL) |
CSFi = CSF positive by immunophenotype methods (immunohistochemical/flow-cytometric analysis) (specify % lymphoma cells) |
CSFc = CSF positive by cytogenetic/FISH analysis (specify % lymphoma cells) |
CSFmol = CSF positive by molecular techniques (PCR-based) (specify level of involvement) |
Note: until sufficient data are available, positron emission tomography (PET) should be used with caution for staging and PET results should be compared and discussed in light of other more consolidated imaging approaches |
Table 4.6
Adult non-Hodgkin staging systems and prognostic index
Ann Arbor classification used in adult non-Hodgkin lymphoma | ||
Stage I | Involvement of a single lymph node region (I) or a single extralymphatic organ or site (IE) | |
Stage II | Involvement of two or more lymph node regions on the same side of the diaphragm (II), which may be accompanied by a contiguous involvement of an extralymphatic organ or site (IIE) | |
Stage III | Involvement of lymph node regions on opposite sides of the diaphragm, which may be accompanied by involvement of the spleen (IIIS) or by a localized involvement of an extralymphatic organ or site (IIIE) or both (IIISE) | |
Stage IV | Disseminated involvement of one or more extralymphatic organ or tissues, with or without associated lymph node involvement | |
International prognostic index used in adult non-Hodgkin lymphoma (patients <60 year) | ||
Factors | Risk classification | |
Performance status >2 | Low | 0 factor |
LDH > normal | Low–intermediate | 1 factor |
Stages III–IV | High–intermediate | 2 factors |
High | 3 factors | |
The IPI has been updated twice, firstly by the Vancouver Group who proposed the Revised IPI (R-IPI) after the introduction of rituximab into adult practice [9] (it is fair to observe that this was not universally accepted [10]) and more recently the new NCCN index that has been proposed [11] (again it is not clear yet how widely this will be adopted). The principal modification in the NCCN index is the introduction of three levels of serum LDH to acknowledge very high values likely to represent very bulky tumors and also a similar subdivision in the scoring for age. This new age scoring system is not relevant to the younger patients discussed in this chapter except that it does emphasize the generally favorable outcomes in all patients under 50 years.
PS does not seem appropriate for very fast-growing tumors such as BL and LL and is often not documented in pediatric lymphoma trials. This might make comparisons difficult between childhood and adult studies, especially in large cell lymphoma. PS should be included in future studies that included adolescents. In spite of being an unspecific marker and of different methods of dosage with different “norms,” serum LDH level is a very good indicator of tumor burden and generally has prognostic significance.
The traditional boundary between leukemia and lymphoma has been defined arbitrarily be more or less than 25 % blast cells in the bone marrow, but this does not correspond to either clinical or biological differences [8]. CNS involvement is defined by the presence of unequivocal malignant cells in a cytocentrifuged specimen of spinal fluid and/or the presence of obvious neurological deficits, such as cranial nerve palsies [8].
Experience with positron emission tomography (PET) in childhood and adolescent NHL is in the early stages of investigation. It is hoped that this diagnostic tool will help to predict the presence of active tumors in a residual mass.
It must be noted that in adult practice by contrast, PET scanning has now been fully established as an essential part of patient management with respect to staging and end of treatment response assessment. This is best illustrated by the revision to the Cheson Response Criteria for Clinical Trials [12, 13] which fully integrates PET/CT scanning into routine research practice and by extension to best routine clinical practice. A much more controversial issue is the use of interim PET scanning to dictate prognosis and therapy. Unlike Hodgkin disease, where this is increasingly well established [14], in DLBLC there is no definite data to support the use of interim PET [15, 16]. Further discussion is beyond the scope of this chapter but in summary there appears to be no improvement in overall outcome when interim PET is used to test whether treatment should be changed during standard therapy. Recently, a new response criterion has been developed for children with NHL entitled “International Pediatric NHL Response Criteria” which incorporates contemporary imaging and laboratory diagnostic testing (Tables 4.7 and 4.8) [17].
Table 4.7
International pediatric NHL response criteria (IPNHLRC)
Complete response (CR): disappearance of all disease (three designations) |
1. Complete (CR): |
(a) CT or MRI reveals no residual disease or new lesions |
(b) Resected residual mass that is pathologically (morphologically) negative for disease (detection of disease with more sensitive techniques described as in “supporting data” as in Table 4.2 |
(c) BM and CSF morphologically free of disease (detection of disease with more sensitive techniques described as in “supporting data” as in Table 4.2) |
2. Complete response, biopsy negative (CRb): |
(a) Residual mass has no morphological evidence of disease from limited or core biopsy (detection of disease with more sensitive techniques described as in “supporting data” as in Table 4.2) with no new lesions by imaging examination |
(b) BM and CSF morphologically free of disease (detection of disease with more sensitive techniques described as in “supporting data” as in Table 4.2) |
(c) No new and/or progressive disease elsewhere |
3. Complete response, unconfirmed (CRu): |
(a) Residual mass is negative by FDG-PET; no new lesions by imaging examination |
(b) BM and CSF morphologically free of disease (detection of disease with more sensitive techniques described as in “supporting data” as in Table 4.2) |
(c) No new and/or progressive disease elsewhere |
Partial response (PR): 50 % decrease in the sum of the product of the greatest perpendicular diameters (SPD) on CT or MRI. FDG-PET may be positive (Deauville score of 4 or 5 with reduced lesional uptake compared to baseline). No new and/or PD. Morphological evidence of disease may be present in the BM or CSF if present at diagnosis (detection of disease with more sensitive techniques described as in “supporting data” as in Table 4.2); however, there should be a 50 % reduction in the percentage of lymphoma cells |
Minor response (MR): decrease in SPD is greater than 25 % but less than 50 % on CT or MRI. No new and/or PD. Morphological evidence of disease may be present in the BM or CSF if present at diagnosis (detection of disease with more sensitive techniques described as in “supporting data” as in Table 4.2); however, there should be a 25–50 % reduction in the percentage of lymphoma cells |
No response (NR): for those who do not meet CR, PR, MR, or PD criteria |
Progressive disease (PD): for those with greater than 25 % increase in the SPD on CT or MRI, Deauville score 4 or 5 on FDG-PET with an increase in lesional uptake from baseline, or the development of new morphological evidence of disease in the BM or CSF |
Table 4.8
Supporting IPNHLRC data
Bone marrow involvement |
BM involvement is currently defined by morphological evidence of lymphoma cells. This applies to any histological subtypes |
Type and degree of BM involvement should be specified, using the abbreviations below: |
BMm = BM positive by morphology (specify % lymphoma cells) |
BMi = BM positive by immunophenotypic methods (histochemical/flow-cytometric analysis) (specify % lymphoma cells) |
BMc = BM positive by cytogenetic/FISH analysis (specify % lymphoma cells) |
BMmol = BM positive by molecular techniques |
Same approach should be used for peripheral blood (PB) involvement (i.e., PBm; PBi, PBc, PBmol) |
Central nervous system (CNS) involvement |
CSF status: CSF positivity is based on morphological evidence of lymphoma cells |
CSF should be considered positive when any number of blasts is detected |
CSF unknown |
Similarly to BM, type of CSF involvement should be described whenever possible |
CSFm = CSF positive by morphology (specify the number of blasts/μL) |
CSFi = CSF positive by immunophenotype methods (histochemical/flow-cytometric analysis) (specify % lymphoma cells) |
CSFc = BM positive by cytogenetic/FISH analysis (specify % lymphoma cells) |
CSFmol = CSF positive by molecular techniques |
Residual mass (RM) |
RMm: tumor detected by standard morphological evaluation |
RMi: tumor detected by immunophenotype methods (immunohistochemical or flow-cytometric analysis |
RMc: tumor detected by cytogenetic/FISH analysis |
RMmol: tumor detected by molecular techniques |
Patients often have other problems at diagnosis, such as malnutrition, infection, postsurgical complications, and respiratory and metabolic abnormalities; these may be life threatening or compromise the onset of therapy. Tumor lysis syndrome (TLS) may be present at diagnosis or may develop during treatment. Cairo et al. developed a classification and grading system for TLS [18]. In advanced diseases, especially in BL and LL, preventive measures must always be institute: hyperdiuresis and “uricolytic” drugs (allopurinol or urate oxidase). Urate oxidase should be utilized in cases of high tumor burden [19–22]. Urate oxidase converts uric acid allantoin, which is highly soluble in urine. It is an efficient way of promptly reducing serum uric acid levels, thus preventing uric acid nephropathy and preserving renal function, allowing a better excretion of the other cell metabolites such a potassium and phosphorus. Strict clinical and metabolic monitoring of patients during the lysis phase is essential.
4.7 B-Cell Non-Hodgkin Lymphoma
The two main entities of NHL are BL and DLBCL. The other B-cell NHL, such as mantle cell, or mucosa-associated lymphoid tissue lymphomas, are not often encountered in adolescents and young adults and will not be discussed in this chapter.
4.7.1 Burkitt Lymphoma
BL is the most common NHL of childhood, adolescents, and young adults (CAYA) [23, 24]. The incidence, however, decreases with age, being highest in children <15 years and relatively uncommon in adults >30 years of age. BL is characterized by very distinct epidemiologic differences. Sub-Saharan Africa has an especially unique epidemiology of childhood cancer where endemic BL is the most common pediatric malignancy overall, representing up to one-third to one-half of all pediatric oncologic diagnoses. Sporadic BL, occurring everywhere else in the world, accounts for approximately 40 % of NHL in CAYA outside of sub-Saharan Africa.
BL is characterized on one hand by a high prevalence (around 70 %) of advanced-stage III–IV disease presentation but on the other hand excellent clinical outcomes with event-free survival (EFS) of 85–90 % overall. Most commonly, BL presents with intra-abdominal masses; however masses of the head and neck also occur. The tumor burden in BL is often exceptionally high, with more than half of patients presenting with a significantly elevated lactate dehydrogenase (LDH) level >2 times the upper limit of normal [25]. With this in mind, it is no surprise that BL carries one of the highest risks for the complication of TLS and close monitoring of patients prior to and during induction therapy is vital [18, 19].
The defining characteristic of BL is a translocation of the C–MYC oncogene on chromosome 8 with the immunoglobulin genes on chromosome 14, 22, or 2. Histology classically reveals intermediate-sized cells with round nuclei and scant cytoplasm with lipid vacuoles. BL has one of the highest proliferation rates of any malignancy and usually reveals numerous mitotic figures and apoptotic bodies that can be seen engulfed in scattered macrophages portraying the characteristic “starry-sky” appearance on low power histology [25]. Endemic BL occurs in the holoendemic malaria belt of sub-Saharan Africa and is virtually always associated with Epstein-Barr virus (EBV) infection. On the other hand, sporadic BL is associated with EBV in about 30 % of cases [26].
The immunophenotypic signature of BL is characterized by expression of mature B-cell antigens CD20 and CD19, as well as CD10 and BCL6, which are associated with germinal center derivation. BL expresses the proliferation antigen Ki-67 at rates often >99 % and rarely expresses BCL2 [27].
Landmark gene expression profiling (GEP) studies have recently established an extensive biologic definition of B-NHL, producing a molecular definition of BL that extended the spectrum of the WHO criteria [28]. Additionally, Dave/Staudt et al. established that C–MYC and its target genes as well as a subgroup of germinal center B-cell genes were more highly expressed in BL versus DLBCL [29]. GEP studies in pediatric B-NHL have highlighted similar findings [30].
The major determinants for risk stratification in BL of CAYA are rooted in the original Murphy stage of the clinical presentation. The previous FAB96 study demonstrated the clinical variables associated with a significantly inferior EFS in pediatric mature B-NHL; those include advanced-stage disease, elevated LDH, primary mediastinal involvement, and combined BM and CNS disease [31]. Additionally, one of the most important prognostic indicators to guide treatment decision is the patient’s response to therapy. On the FAB/LMB protocol, failure to achieve at least 20 % reduction in disease burden with the first week of reduction phase chemotherapy is a poor prognostic indicator and requires intensification of the treatment regimen [32]. Additionally, failure to achieve complete remission (CR) by the completion of the first consolidation cycle is also associated with poor long-term survival and is another indication to intensify therapy. Universal guidelines to evaluate treatment response via fludeoxyglucose (FDG)-PET for NHL in CAYA have been published and will serve as the benchmark for further delineating the role of FDG-PET through prospective clinical trials [8, 33].
The prognostic role of BL biology with regard to cytogenetic and minimal-residual disease (MRD) findings is currently being explored. Although specific cytogenetic findings such as deletion 13q, gain of 7q, and complex cytogenetics may be associated with a higher risk for treatment failure, further studies are needed to determine if these patients require and/or benefit from an intensification of the therapeutic approach [34, 35]. Preliminary data on the presence of MRD in B-cell NHL suggested that the presence of MRD on end-of-therapy (EOT) specimens was associated with disease recurrence [36]. Children/adolescents with intermediate-risk B-NHL, treated with FAB/LMB96 modified chemotherapy plus rituximab, had blood and bone marrow specimens from end of induction (EOI) and EOT assessed for MRD [36]. While recurrence rates were similar between the EOI MRD-positive and MRD-negative patients (p = 0.40), EOT MRD results demonstrated one relapse in the MRD-positive group and no recurrences in the MRD-negative group (p = 0.077). More recent data compared the presence of MRD from EOI and end of consolidation, demonstrating that the presence of MRD did not predict relapse and that subsequent therapy actually appeared to eliminate MRD [37]. Further prospective investigations will determine the prognostic role of MRD in BL in CAYA.
Long-term curative outcomes in pediatric BL have dramatically improved over the past three decades, with EFS rates essentially doubling from the late 1970s to the contemporary era (Fig. 4.6) [38]. Since the turn of the century, clinical trials have focused on establishing risk-stratified therapy to diminish acute and long-term toxicities for patients with favorable prognosis and to intensify regimens for those with higher risk for treatment failure [32, 39–41]. International collaboration in large-scale clinical trials has resulted in the modern day FAB/LMB chemotherapy backbone consisting of cyclophosphamide, high-dose methotrexate (HD MTX), cytarabine, vincristine, doxorubicin, and corticosteroids.
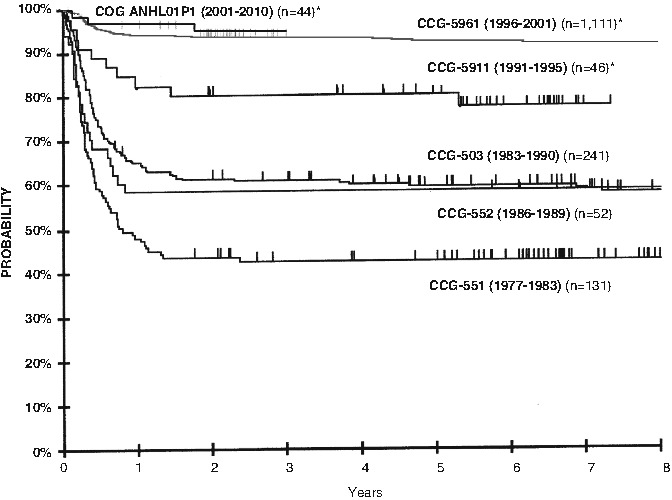

Fig. 4.6
Comparison of EFS by CCG study among stage III/IV and BM/CNS Burkitt and Burkitt-like lymphoma patients from CCG/COG studies: C551, C503, C552, C5911, C5961, and ANHL01P1. CCG Children’s Cancer Group, COG Children’s Oncology Group, EFS event-free survival (Modified and used with permission from Wiley: Cairo et al. [38], copyright (2003))
FAB/LMB chemotherapy protocols were established based upon three risk-stratification groups. Low-risk group A patients with fully resected stage I or abdominal stage II disease are treated with two cycles of systemic chemotherapy (without intrathecal), with 4-year EFS rates of 98.3 % [42]. FAB group B intermediate-risk regimens demonstrated equivalent EFS rates of 91 % with reduced total doses of cyclophosphamide and deletion of maintenance cycles [32]. Even patients with group C high-risk disease—defined as having central nervous system (CNS) involvement and/or >25 % bone marrow involvement—achieve long-term EFS rates of 79 % with the FAB/LMB backbone chemotherapy bolstered by the addition of HD MTX (8 g/m2 for CNS-positive patients) during induction and cytarabine (12,250 mg/m2) plus etoposide for two cycles of intensification followed by four cycles of maintenance chemotherapy [39]. The Berlin-Frankfurt-Munster (BFM) collaborative group uses a very similar risk-stratification system with four groups instead of three; FAB group B is essentially broken down into two groups in the BFM approach. Similarly, their treatment regimen is nearly the same, with slight variations in chemotherapy dosing, number of cycles, and the addition of ifosfamide in the BFM approach accounting for the nuanced differences (see Table 4.9) [25].
Table 4.9
Comparison of risk-stratification and treatment regimens between FAB and BFM protocols for CAYA with mature B-NHL
Group | BFM R1 | FAB group A | BFM R2 | FAB group B | BFM R3 | BFM R4 | FAB group C |
|---|---|---|---|---|---|---|---|
Definition | Resected | Resected | Not resected I, II, III-LDH <500 | Not resected I, II, III—CNS negative | III-LDH 500–999 IV+ B-ALL LDH <1000, CNS negative | LDH >1000 and/or CNS positive | B-ALL IV—CNS positive |
No of courses | 2 | 3 | 4 | 4 | 5 | 6 | 8 |
MTX g/m2, infusion | 1, 4 h, ×2 | – | 1, 4, h, ×4 | 3, 3 h, ×4 | 5, 24 h, ×4 | 5, 24 h, ×4 | 8, 4 h (CNS+; 24 h) |
Dox mg/m2 | 50 | 120 | 100 | 120 | 100 | 100 | 240 |
CP g/m2 | 1–2 | 3 | 2–4 | 3–3 | 2–4 | 2–4 | 6–8 |
Ifo g/m2 | 4 | – | 8 | – | 8 | 8 | 0 |
Eto mg/m2 | 200 | – | 400 | – | 900 | 1400 | 2500 |
Recent large-scale prospective data from the FAB/LMB96 study demonstrated that adolescent age was not associated with a risk of treatment failure in comparison to children with BL [31]. Historically though, there had previously been a general consensus that adolescents with mature B-cell lymphomas experienced inferior outcomes based upon data from the United States, Germany, and France [38, 43, 44]. However, this data may be clouded by multiple factors including derivation from retrospective analyses spanning decades (CCG and BFM data) and analysis of smaller numbers of patients (SFOP data) and that all three studies occurred prior to the discovery of primary mediastinal B-cell lymphoma as a separate B-NHL diagnostic entity requiring different therapy that tends to occur in adolescents and young adults. It is currently accepted that adolescent age is not prognostically significant in BL.
While adults with BL have always experienced much lower curative rates than pediatric patients, this has been attributed to the intrinsically different treatment approaches utilized. More recently, adult BL clinical trials have incorporated pediatric-style chemotherapy regimens with encouraging success [45]. In general, it is well established that pediatric BL protocols achieve significantly higher curative outcomes for the following reasons: (1) dose intensity is incorporated by utilizing 21 rather than 28-day cycles, (2) HD MTX is uniformly given, and (3) group C patients receive both HD MTX and high-dose cytarabine. Historically, when treated on the same protocol, children and young adults achieve similar outcomes [46, 47]. Figure 4.7 demonstrates survival curves for children and adolescents treated on the FAB/LMB pediatric regimen [31, 45]. Table 4.10 shows a comparison of the most recent survival data for CAYA treated on large-scale prospective clinical trials utilizing FAB/LMB backbone chemotherapy regimens.
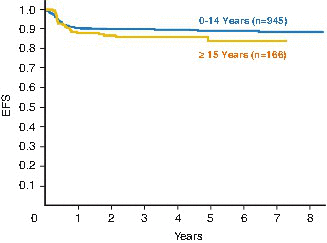

Fig. 4.7
Three-year probability of event-free survival in children (ages 0–14 years) versus adolescents (ages 15–21 years) treated in the French-American-British mature B-cell lymphoma 96 (FAB/LMB 96) study (Reprinted with permission. © (2012) American Society of Clinical Oncology. All rights reserved.” Cairo et al. [30])
Table 4.10
Comparison of survival outcomes comparing children, adolescents, young adults, and adults treated on large-scale prospective protocols
Cairo et al. [31] | Hoelzer et al. [45] | ||||
|---|---|---|---|---|---|
0–14 years | 15–21 years | 15–25 years | 26–55 years | >55 years | |
Number of patients | 945 | 166 | 69 | 196 | 98 |
EFS/PFS | 89 % | 84 % | 82 % | 60 % | |
Overall survival | 91 % | 85 % | 90 % | 84 % | 62 % |
The vast majority of BL cases in CAYA express the CD20 antigen, begging the question of whether adding anti-CD20-targeted immunotherapy to standard chemotherapy will improve outcomes [48]. Adding rituximab to BL chemotherapy has been shown to be safe and effective in the largest prospective trial for adults with BL, with particularly excellent outcomes for AYA [45]. The Children’s Oncology Group (COG) recently published results on a pilot study for mature B-NHL integrating rituximab into the standard FAB/LMB backbone chemotherapy. The study showed that it was safe to combine rituximab with FAB/LMB chemotherapy, and this chemoimmunotherapeutic approach yielded a 95 % probability of EFS at 3 years for 45 patients with advanced-stage III/IV group B intermediate-risk disease [40]. Meanwhile, rituximab combined with group C regimen produced a 3-year EFS of 90 % in 40 patients [41]. The BFM also evaluated the efficacy of rituximab with 37 of 87 newly diagnosed patients with B-NHL (42 %) demonstrating a significant response to a 1-week window of a single dose of rituximab [49]. Current large-scale clinical trials in pediatric BL are focusing on evaluating the combination of rituximab plus FAB/LMB chemoimmunotherapy regimen in a larger cohort of patients [50].
Although the prognosis for patients with newly diagnosed BL is excellent, CAYA with relapsed and/or refractory BL experience dismal outcomes. With reported long-term survival rates ranging from 12 % to 28 %, it is well established that salvaging those patients with relapsed/refractory BL is inordinately difficult (Fig. 4.8) [38, 39, 51]. A reinduction regimen combining ifosfamide, carboplatin, and etoposide (ICE) with rituximab for children with relapsed/refractory B-NHL produced encouraging results; however maintaining long-term remission can only be established utilizing hematopoietic stem cell transplantation (HSCT) [52, 53]. While the data for HSCT in relapsed/refractory BL is not robust, it has been demonstrated repeatedly that long-term survival utilizing high-dose chemotherapy followed by autologous HSCT is <30 % [54, 55]. The combination of autologous HSCT followed in tandem by reduced-intensity allogeneic HSCT to maintain long-term remission has shown promise in small numbers of CAYA with relapsed/refractory BL; five of eight patients achieved long-term CR (1.9–8.8 years) utilizing this approach [56]. There seems to be a potential graft-versus-lymphoma (GVL) effect in BL and it is vital to further determine the potential role of allogeneic HSCT in CAYA with relapsed/refractory BL.
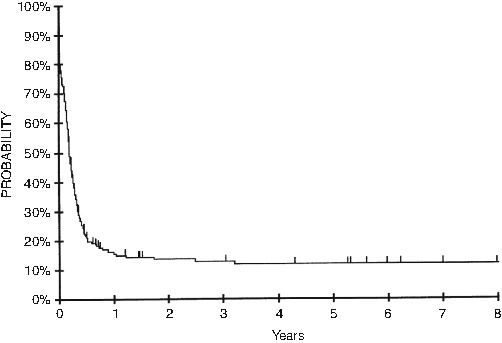

Fig. 4.8
Survival probability after progression/recurrence of mature B-NHL in CAYA. The probability of 4-year survival of patients with persistent, progressive, or recurrent disease, excluding those experiencing toxic deaths or secondary neoplasms. This research was originally published in Blood (Cairo et al. [39]. © by the American Society of Hematology)
Certainly long-term survival in patients with relapsed or refractory BL in the modern era is meager. In the absence of established therapies with curative potential, novel approaches to therapy are desperately needed. Therapeutic strategies that are currently under investigation include agents targeting specific molecular pathways and novel cellular and humoral immunotherapies. Promising targeted cell-signaling pathway inhibitors include ibrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor, and idelalisib, a phosphoinositide-3-kinase inhibitor [57, 58]. Chimeric antigen receptor-based cellular immunotherapies are under evaluation in clinical trials for CAYA based upon exciting results from preclinical data as well as success in treating acute lymphoblastic leukemia (ALL) [59, 60]. Finally, novel humoral immunotherapies targeting both CD19 (blinatumomab and SAR3419) and CD20 (obinutuzumab and ofatumumab) are also being evaluated in clinical trials. As a novel anti-CD20 monoclonal antibody that has demonstrated superior results in comparison with rituximab in both preclinical and clinical trials, obinutuzumab is currently being evaluated in a clinical trial for CAYA with relapsed/refractory mature B-NHL in combination with the reinduction ICE chemotherapy platform (NCT02393157) [61–65]. With a number of novel therapeutic agents on the horizon, there is hope to not only improve the chances for salvaging patient with relapsed/refractory disease but to also improve overall outcomes by incorporating targeted therapies with chemotherapy in up-front regimens.
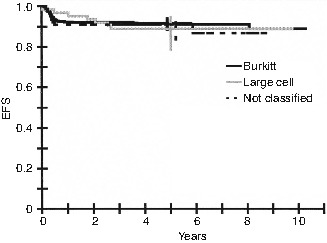
4.7.2 Diffuse Large B-Cell Lymphoma
Depending on the country in pediatric practice, DLBCL is included either in studies designed for BL (LMB and BFM studies) [44, 66] or in studies designed for large cell NHL in general (Pediatric Oncology Group [POG] studies) [67, 68]. In the LMB89 study, DLBCL represented 10 % of all registered patients. As with BL, they are treated according to initial resection and stage. The EFS is similar to that of BL (Fig. 4.9), but it should be noted that the proportion of patients with advanced stages is lower than in BL. However, by stage, EFS is not significantly different [69]. In the BFM90 study, the EFS of DLBCL is also similar to that of BL. The criticism of such an approach is that too much CNS-directed therapy is given in DLBCL, in which the risk of CNS disease is lower than in BL.