Fig. 4.1
A 66-year-old male with advanced metastatic CRPC on FDG PET/CT demonstrates an FDG-avid (a) right paratracheal metastatic lymph node and (b) vertebral body bone metastases (T3, T12, L1)
Sodium Fluoride: Bone Remodeling
The main sites of prostatic skeletal metastases are ribs, thoracic and lumbar vertebrae, and ilium of the pelvis. Other less common sites of involvement are ischium, sacrococcyx, femur, skull, cervical vertebrae, sternum, scapula, and upper limb bones [21]. The bone involvement is commonly detected by BS and standard radiographs. Other imaging modalities such as CT, MRI, PET, and PET/CT can also be used in specific circumstances based on imaging timing, costs, radiation dose, and availability [22]. The current sequential approach is BS, BS plus TXR, and addition of CT or MRI in equivocal or discrepant cases [23].
18F-Sodium Fluoride (NaF) is gaining renewed interest and wider application for detection of osseous metastatic disease because of the widespread availability and improved quality of PET/CT scanners, but also because of the intrinsically higher spatial resolution of PET (compared to planar and SPECT imaging) and potential for PET quantitation of metastatic tumor burden [24]. NaF is a marker of bone perfusion and bone turnover, in which 18F-Fluoride ions exchange with hydroxyl groups in the hydroxyapatite crystal of bone to form fluorapatite with higher uptake in new bone (osteoid) because of higher availability of binding sites [24]. NaF PET/CT has been reported to be a highly sensitive and specific modality for detection of bone metastases in prostate cancer compared to standard 99mTc-MDP (MDP) planar bone scans and MDP SPECT imaging [25]. The anatomic CT portion of the PET/CT was able to differentiate between benign and malignant lesions improving the specificity of NaF PET/CT vs NaF PET alone. Recent clinical practice guidelines for NaF PET/CT bone scans have been published by the Society of Nuclear Medicine and Molecular Imaging in 2010 [26]. A systemic review of the literature provides evidence for superior detection of bone metastases by both NaF PET and a functional tumor-based choline PET imaging agent (18F-fluorocholine or 11C-choline), with or without CT, compared with conventional planar MDP bone scan [27]. This review reported a sensitivity and specificity for NaF PET or PET/CT of 88.6 and 90.7 %, respectively, on a per lesion basis, and 86.9 and 79.9 % on a per patient basis. It is noted that NaF uptake post-treatment flare phenomenon, similar to that observed with MDP bone scans, has also been observed, which should be taken into consideration [28]. Figure 4.2 shows examples of NaF PET/CT bone metastases and benign uptake in a degenerative vertebral osteophyte.
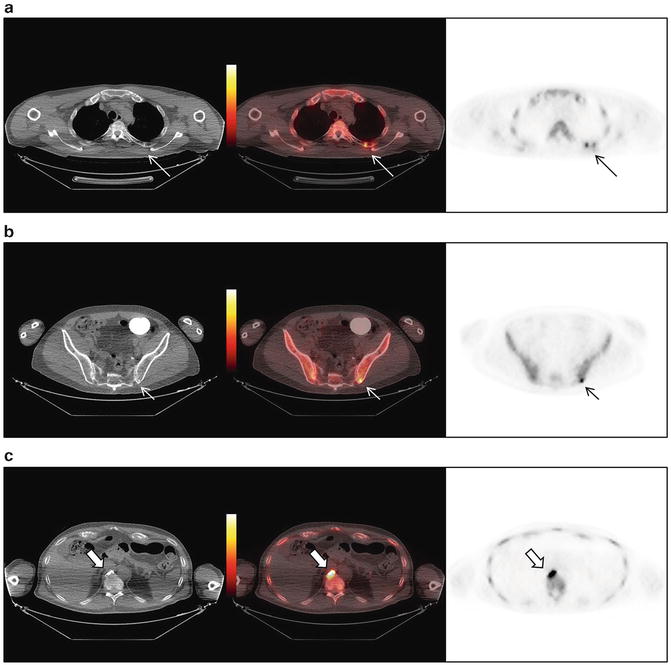

Fig. 4.2
A 68-year-old male with bone metastases on NaF PET/CT involving the (a) left fourth rib (long arrow), (b) left posterior iliac bone (short arrow), and (c) additional site of benign uptake at a degenerative vertebral body endplate osteophyte (arrow head)
One of the benefits of NaF PET compared to 99mTc-MDP (MDP) bone scans is the potential for quantitation of bone metastatic tumor burden and treatment response. A bone scan index using standard planar MDP bone scan had been proposed as an imaging biomarker to improve the reproducibility of treatment response assessment [29]. This index aimed to quantify tumor burden as a percentage of the total skeletal mass of a reference man but there are inherent limitations based on planar imaging. PET imaging with NaF for quantitative analysis for therapy response assessment would have potentially improved benefits over more limited planar and visual qualitative MDP bone scan-based assessment methods. One report in the literature using NaF PET for monitoring treatment response of bone metastases to radiotherapy 223Ra-Chloride was found to be more accurate than the visual qualitative assessment of scans, correlation with the serum-based PSA response or alkaline phosphatase activity [30]. A study of the kinetics and reproducibility of NaF PET/CT for detection and quantitation of bone metastases concluded an uptake period of 60 ± 30 min was sufficient for quantitation, with limited temporal dependence and demonstrated high tumor-to-normal tumor ratio [31]. Repeated baseline scans in this study also showed high intraclass correlations (>0.9) and relatively low critical percentage change (the value above which a change can be considered real) for these parameters, demonstrating relatively high reproducibility.
Lipid Metabolism
Choline
Radiolabeled choline PET imaging radiotracers, 11C-Choline and 18F-Fluorocholine, are taken up in prostate cancer cells through choline transporters and phosphorylated intracellularly by choline kinase, associated with phospholipid metabolism [32–35]. 11C-Choline demonstrates rapid prostate cancer uptake, rapid blood pool clearance (within minutes), relatively small excretion in urine and relatively high diffuse liver uptake [36]. 18F-Fluorocholine demonstrates higher urinary excreted radiotracer compared to 11C-Choline but has the advantage of longer half-life allowing for wider clinical application through established regional radiosynthesis distribution networks without a need for an on-site cyclotron and radiochemistry facility at non-academic sites [37]. Both 11C-Choline and 18F-Fluorocholine have been used widely for a variety of prostate cancer imaging applications, mostly in Europe and Japan, in a large number of publications. Recently, 11C-Choline was approved by the US Food and Drug Administration (FDA) in 2012 to the Mayo Clinic in Rochester, Minnesota, for production and PET imaging of patients with suspected recurrent prostate cancer after initial therapy based on elevated serum prostate specific membrane antigen (PSA) levels and noninformative BS, CT, or MRI imaging to help localize potential sites of tumor for subsequent histologic confirmation [38].
There have been several recent systematic reviews which help to compile and summarize potential clinical applications. Overall, 11C-CholinePET/CT has been reported to affect therapy management in 24 % (11/45) of patients with advanced prostate cancer [39], and generally 11C-Choline and 18F-Fluorocholine have similar performance for detection of prostate cancer in various settings with more detailed analyses below and also reviewed [13].
The diagnostic performance of 18F-Fluorocholine or 11C-Choline PET or PET/CT for staging at initial diagnosis for prostate cancer has been compiled in a systematic literature review and meta-analysis by Evangelista et al. in 2013. They reported low sensitivity but high overall specificity for detection of LNM prior to prostatectomy. In their analysis of 10 selected studies, a total of 441 patients from 2000 to January with pooled sensitivity of 49.2 % (95 % confidence interval [CI], 39.9–58.4) and pooled specificity of 95 % (95 % CI, 92–97.1) was reported [40]. A major issue for the low sensitivity of these choline-based radiotracer is reportedly due to the inherent limitation of PET imaging to detect small metastatic lymph nodes less than 0.4 cm in diameter.
The utility of 18F-Fluorocholine or 11C-Choline PET or PET/CT for detection of prostate cancer recurrence after definitive radical prostatectomy or external-beam radiation therapy was also compiled in another systematic literature review and meta-analysis by Evangelista et al. in 2013 [41]. This study found a high sensitivity and specificity for detection of locoregional and distant metastases by choline PET or PET/CT imaging. Through their selection criteria, 19 studies were chosen with a combined total of 1,555 patients from 2000 to 2012 with a pooled data demonstrating for all sites of disease (prostatic fossa, lymph nodes, and bone) a sensitivity of 85.6 % (95 % CI: 82.9–88.1 %) and specificity of 92.6 % (95 % CI: 90.1–94.6 %), for prostatic fossa recurrence a sensitivity of 75.4 % (95 % CI: 66.9–82.6 %) and specificity of 82 % (95 % CI: 68.6–91.4 %), and for LNM a sensitivity of 100 % (95 % CI: 90.5–100 %) and specificity of 81.8 % (95 % CI: 48.2–97.7 %) [41]. They conclude that choline-based PET or PET/CT imaging can be used for the identification of lymph node recurrence, but raise concerns that due to the loss in specificity may result in unnecessary surgical treatments. The authors recommend identification of relapse in prostate cancer patients based on PSA stratification, with the strongest predictor of PET positivity based on threshold PSA-based parameters (PSA > 1 ng/mL, PSA velocity (vel) > 1 ng/mL/year, and a PSAdt < 3 months). Ongoing hormonal therapy did not limit the diagnostic accuracy for detection of metastatic disease. However, they do not recommend choline PET/CT for detection of local recurrence [41].
Another systematic review and meta-analysis of 18F-Fluorocholine or 11C-Choline PET or PET/CT for staging and restaging of prostate cancer was performed by Umbehr et al. [42]. They reviewed the literature up to July 2012 and selected 44 studies. The authors could not recommend Choline PET or PET/CT imaging without reservation for routine clinical use for prostate cancer imaging based on current evidence, although the diagnostic evidence was found to be higher in restaging than in staging settings. They also recommend careful selection of eligible patients based on PSA criteria to avoid false negative imaging results up front in staging as well as restaging clinical scenarios. In the staging scenario, mainly high-risk Gleason scores (8–10) and high PSA levels (≥20 ng/mL) were thought to be predictive of improved choline PET and PET/CT imaging performance. In restaging settings, minimal recurrent PSA levels (>1 ng/mL), short PSAdt (<3 mo to a maximum of 6 months), and initial tumor stage (>pT3b or pN1) were found to improve imaging detection [42].
Acetate
Acetate is another lipid-based agent incorporated into prostate cancer cells with over-expression of fatty acid synthase, a key enzyme in fatty acid synthesis from acetyl CoA [43]. 11C-Acetate as a PET radiotracer was first demonstrated to detect primary prostate cancer and metastatic disease at initial staging in patients with rising PSA after radical prostatectomy [44] or after external-beam radiation therapy [45].
A systematic review of the literature with meta-analysis of 11C-Acetate PET imaging in prostate cancer was performed by Beheshti et al. [46]. They reviewed all published studies up to March 2013 and selected 24 studies for meta-analysis. For primary tumor detection, pooled sensitivity 75.1 % (95 % CI: 69.8–79.8) and specificity was 75.8 % (95 % CI: 72.4–78.9). For detection of recurrence tumor, pooled sensitivity was 64 % (95 % CI: 59–69) and specificity was 93 % (95 % CI: 83–98), with higher sensitivity in patients with PSA > 1 ng/mL and in post-prostatectomy compared to external-beam radiation therapy patients [46]. Of note, studies comparing 11C-acetate and Choline-based PET imaging were reported to be comparable in their analyses with low sensitivity and relatively high specificity for detection of tumor recurrence and limited value for detection of primary tumor.
When 11C-Acetate was compared with MRI and prostatectomy histopathological correlation, 11C-acetate PET/CT demonstrated higher uptake in tumor foci than in normal prostate tissue but PET uptake was not able to distinguish tumor from benign prostate hyperplasia nodules. In a sector-based comparison with histopathology, all tumors greater than 0.5 cm by histopathology revealed sensitivity of 61.6 % and specificity of 80.0 % for 11C-acetate PET/CT, vs sensitivity of 82.3 % and specificity of 95.1 % for multiparametric MRI (T1, T2, DWI, MRS), with the accuracy of 11C-acetate comparable to that of MRI only when tumors were greater than 0.9 cm were assessed [47].
A recent large trial evaluated 11C-Acetate PET/CT prior to prostatectomy for nodal staging compared with pathologic nodal status and clinical follow-up for treatment failure in 107 men with intermediate- or high-risk localized prostate cancer [48]. 11C-Acetate was positive for local pelvic nodal or distant metastatic disease in 33.6 % of patients, with lymph node metastasis present histopathologically in 23.4 % of these PET-positive metastatic patients. The overall performance of 11C-Acetate for detection of lymph node metastasis prior to prostatectomy revealed a sensitivity of 68.0 %, specificity 78.1 %, positive-predictive value of 48.6 %, and negative-predictive value of 88.9 %. Interestingly, 11C-acetate PET detection of any metastasis in this clinical study also independently predicted treatment-failure–free survival in a multivariate analysis [48].
Androgen Receptor Imaging: FDHT
16β-18F-fluoro-5α-dihydrotestosterone (18F-FDHT) is an analog of dihydrotestosterone, the primary ligand for the androgen receptor (AR) which allows for non-invasive PET imaging of AR expression [49]. The first study of seven patients with progressive metastatic CRPC demonstrated 18F-FDHT detection of 78 % of the lesions compared to conventional imaging with average lesion SUVmax value of 5.28 [50]. Another study also demonstrated 18F-FDHT uptake at sites of metastatic disease, confirming AR-mediated 18F-FDHT PET signal as seen with interval decreased 18F-FDHT uptake from baseline (SUVmax decreased 9–70 %) after competition with flutamide [51].
The potential role of AR imaging as a pharmacodynamic marker in prostate cancer has been demonstrated in several recent studies. 18F-FDHT and 18F-FDG PET/CT imaging of a subset of 22 patients enrolled on a phase 1–2 study of MDV3100 (Enzalutamide) was able to show a reduction in 18F-FDHT tumor uptake from baseline (percent SUVmax change range from 20 to 100 %) after starting treatment compatible with competition or blocking of 18F-FDHT from the AR-binding site by the therapeutic agent [52]. However, these patients show a poor treatment response as only 45 % of these patients by 18F-FDG PET/CT scans had 25 % or greater decrease in the FDG PET SUVmax after 12 weeks of therapy. These results position 18F-FDHT as a pharmacodynamic marker of AR binding rather than a therapy response biomarker. A more recent study also utilized 18F-FDHT-PET/CT imaging to measure pharmacodynamic response in a phase I clinical trial of a novel anti-androgen ARN-509 to incorporate imaging to visualize and quantitatively assess by SUV analysis the heterogeneity of AR-binding sites of metastatic disease and determine maximal AR inhibition by the study drug [53]. A clinically applicable method using 18F-FDHT PET quantitative marker as a surrogate of pharmacokinetic parameter to non-invasively assess free AR concentration has been proposed for validation studies in clinical trials [54].
Amino Acid-Based Imaging: FACBC
An emerging and promising PET radiotracer for prostate cancer is anti-1-amino-3-18F-fluorocy-clobutane-1-carboxylic acid (anti-18F-FACBC), a synthetic l-leucine amino acid analog. This agent is taken up in prostate cancer cells by amino acid transporters system (ASC transporter 2 or ASCT2) and to a lesser sodium-coupled neutral amino acid transporters but not incorporated into proteins intracellularly [55]. The initial first-in-human clinical study of anti-18F-FACBC PET/CT demonstrated positive uptake in primary prostate and metastatic disease [56]. Figure 4.3 demonstrates an example of anti-18F-FACBC PET/CT bone and nodal metastasis. In the subsequent clinical study, comparison of anti-18F-FACBC with 111In-capromab pendetide (ProstaScint™) for detection of recurrent disease in 50 men after prostatectomy or external-beam radiation therapy for prostate carcinoma revealed anti-18F-FACBC PET/CT was more sensitive than 111In-capromab pendetide SPECT/CT for detection of recurrent disease, especially for detection of extraprostatic recurrence. For disease detection in the prostate bed, anti-18F-FACBC had a sensitivity of 89 % (95 % CI: 74–97 %), specificity of 67 % (95 % CI: 35–90 %), and accuracy of 83 % (95 % CI: 70–93 %). For the detection of extraprostatic recurrence, anti-18F-FACBC had a sensitivity of 100 % (95 % CI: 69–100 %), specificity of 100 % (95 % CI: 59–100 %), and accuracy of 100 % (95 % CI: 80–100 %). A recent small study compared anti-18F-FACBC with 11C-Choline PET/CT imaging for detection of recurrent disease in 15 men after definitive therapy with prostatectomy or external-beam radiation therapy. Anti-18F-FACBC was found to have a higher detection rate compared to 11C-Choline on a per patient (detection rate of 40 vs 20 %, respectively) and per lesion basis (6 vs 11 lesions, respectively), with all 11C-Choline positive lesions also identified by anti-18F-FACBC [57]. These promising initial studies will need further validation of anti-18F-FACBC in larger multi-center clinical trials.
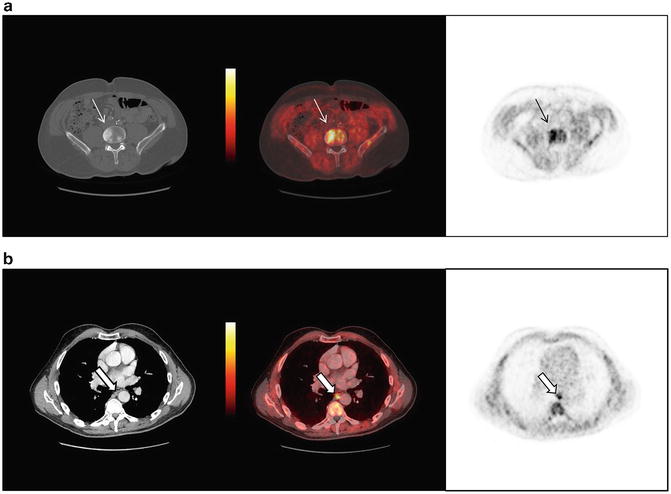

Fig. 4.3
A 64-year-old male with metastatic CRPC imaged with anti-18F-FACBC PET/CT demonstrates (a) a L4 vertebral body bone metastasis and (b) small paraesophageal nodal metastasis
Prostate Specific Membrane Antigen-Based Imaging: PSMA
Prostate specific membrane antigen (PSMA) is a type II integral membrane protein expressed on the surface of prostate cancer cells, also known as glutamate carboxypeptidase II (GCPII) and folate hydrolase [58]. PSMA is associated with prostate cancer aggressiveness with histological studies associating high PSMA expression with metastatic spread [59–61], androgen-independence [62], and high PSMA expression levels predictive of prostate cancer progression [63, 64]. 111In-capromab pendetide (ProstaScint™) was first developed for PSMA-based prostate cancer imaging but demonstrated limited performance for tumor detection which may be explained by lower tumor penetration of a large sized antibody agent and binding to the less accessible intracellular epitope of PSMA [65]. An improved humanized intact antibody that binds to the more accessible extracellular epitope of PSMA, J591, has been used labeled Indium-111 and Lutetium-177 for gamma planar and SPECT/CT imaging of metastatic prostate cancer as part of a number of radioimmunotherapy trials [66–70]. A recent retrospective review of these trials presented as a research abstract ranging over a decade demonstrated J591 planar imaging reported a detection rate of 86.4 % of known lesions [67]. Next generation imaging trials are incorporating PET/CT imaging of prostate cancer with 89Zr-DFO-J591 which demonstrated high prostate tumor uptake in preclinical models [71].
Smaller low molecular weight imaging agents for PSMA would have inherent advantages over intact antibodies such as rapid tumor uptake and clearance from non-target sites [58]. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-18F-fluorobenzyl-l-cysteine (18F-DCFBC) is a novel and clinically practical low molecular weight inhibitor of PSMA which in preclinical studies demonstrated high specific localization to PSMA-expressing prostate cancer xenografts [72]. An initial first-in-man study of 18F-DCFBC PET/CT was able to demonstrate uptake at sites of bone and lymph node metastatic disease detected at two hours after injection, with 18F-DCFBC PET detecting more lesions than corresponding conventional imaging modalities (CT, bone scan) [73]. These promising findings are undergoing further validation in ongoing clinical trials evaluating 18F-DCFBC PET/CT in primary and metastatic prostate cancer. Figure 4.4 demonstrates examples of positive detection of metastases by 18F-DCFBC PET/CT in men with CRPC seen with widespread bone and another with a large left pelvis nodal metastasis. Another fluorine-18 labeled low molecular weight PSMA targeted PET radiopharmaceutical, BAY 1075553, has been published recently with preclinical validation [74].
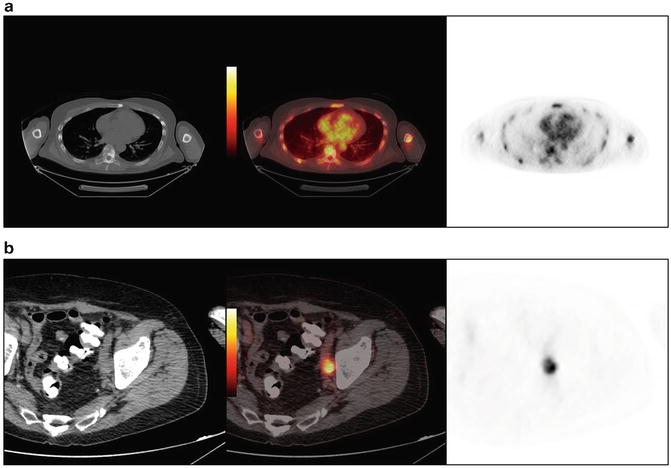

Fig. 4.4
PSMA-based 18F-DCFBC PET/CT demonstrates (a) widespread bone metastases and “superscan” bone scan in a 62-year-old with metastatic CRPC, and (b) activity in a left pelvic side-wall lymph node metastasis in a 74-year-old male with CRPC
Other radiolabeled PSMA-based radiotracers for PET imaging including a Gallium-68 labeled PSMA-based low molecular weight radiopharmaceuticals have also been radiosynthesized [75]. Afshar-Oromieh et al. reported their initial experience with PET/CT using Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)] (68Ga-PSMA) which also was able to detect prostate carcinoma relapses and metastases at high signal-to-background at 1 h after radiopharmaceutical administration [76]. Another recent study comparing 68Ga-PSMA with 18F-Fluorocholine with biochemical relapse of prostate cancer in the same patients within a 10-day time window in 37 patients was able to detect more lesions and higher signal-to-background by 68Ga-PSMA compared to 18F-Fluorocholine (78 vs 56, respectively; P = 0.04) [77].
PSMA-based radiotracers have also been developed for SPECT imaging, which have lower resolution compared to PET scans but are more widely available than PET. First-in-man study of two Iodine-123 labeled small molecule SPECT agents for PSMA, 123I-MIP-1072, and 123I-MIP-1095 also demonstrated uptake and detection of prostate cancer in soft tissue, bone, and the prostate gland as early as 1–4 h after injection [78]. Other SPECT agents for PSMA have been radiosynthesized with technetium-99 m demonstrating high specific PSMA targeted tumor uptake in preclinical models [79–81].
An interest and potentially powerful application of PSMA imaging is as a downstream marker of androgen-receptor signaling to potentially assess for real-time assessment of tumor response to androgen deprivation therapy. Evans et al. reported in preclinical studies that PSMA can be repressed by androgen treatment in multiple animal models of AR-positive prostate cancer in an AR-dependent manner, whereas anti-androgens can up-regulate PSMA expression [82]. In another study, assessment of androgen-receptor signaling in circulating tumor cells was able to utilize PSMA and PSA expression as markers of hormonally responsive prostate cancer to androgen deprivation therapy [83]. With the emergence of PSMA-based radiopharmaceuticals the possibility of utilizing non-invasive PSMA imaging by PET/CT or SPECT/CT as a downstream biomarker of androgen-receptor signaling may be an exciting possibility but requires careful clinical validation.
Magnetic Resonance Imaging
MRI Techniques
Morphologic MR Images
Modern MRI of the prostate applies the principles of multiparametric MRI (mMRI) utilizing morphological imaging (T1-weighted and T2-weighted) and functional imaging (diffusion, perfusion, and spectroscopy). Morphologic MR images provide anatomical detail of the prostate and local tumor staging through T2-weighted images (T2WI), and some information about presence of post-biopsy hemorrhage and lymph node involvement through T1-weighted images (T1WI). In the untreated gland, peripheral zone (PZ) tumor can be detected as low signal intensity (SI) (dark) lesion on T2WI. However, there are some conditions like prostatitis, biopsy scars or fibrosis, and treatment-induced changes that may mimic the PZ tumor or may mask the surrounding or intermixed tumor.
Detection of transition zone (TZ) tumor is challenging in T2WI because of the signal heterogeneity secondary to benign prostatic hyperplasia (BPH). The TZ tumors are typically recognized as homogeneously low SI lesion on T2WI in the absence of the low SI rim surrounding the BPH nodules. Moreover, stromal type BPH and high-grade prostatic intraepithelial neoplasia (HGPIN) may also appear similar to TZ tumors on T2WI. Also, detection of anterior TZ tumors is difficult using T2WI alone due to close proximity to anterior fibromuscular stroma [84].
Diffusion-Weighted Imaging
The study of water diffusivity in the tissue is crucial to identify the tumor lesions based on different water content and cellular density. Cancer cells induce high-density cellular microenvironment and disorganized extracellular stroma appearing as high SI on high b-value DWI. The Apparent Diffusion Coefficient (ADC) value as a measure of water diffusion on DWI is decreased in tumor regions due to water molecule motion restriction. DWI can also be helpful to differentiate prostatitis from tumor tissue. In addition, ADC value is negatively correlated to the tumor grade, the more cellular the tumor, the higher the restriction [85, 86].
Dynamic Contrast-Enhanced MRI
Dynamic contrast-enhanced (DCE)-MRI examines the perfusion and distribution of intravenously administered contrast agent. Tumors exhibit neovascularization secondary to high level of vascular endothelial growth factor (VEGF) expression. The quantitative parameters of DCE-MRI reflect features of tumor vascularity such as disorganized structure, arteriovenous shunting, and areas of hemorrhage [87].
Tumor tissues with increasing cellularity have smaller volume of interstitial space accounting for different pharmacokinetics for distribution of contrast agent; tumor tissues have higher and earlier enhancement, and quick washout of the contrast compared with the healthy prostate gland [88]. Quantitative study of DCE-MRI with pharmacokinetic (PK) modeling can quantify the tumor blood flow, tumor microvasculature, and capillary permeability [89].
MR Spectroscopic Imaging
Metabolic profile of tumor differs from the healthy tissue on spectroscopic imaging. The main metabolites appearing on MR spectroscopic imaging (MRSI) of the prostate are choline, creatine, and citrate. The citrate level represents the glandular component of the respective voxel. Citrate level is higher in the normal PZ and glandular type of BPH than in the stromal type of BPH. The citrate level is decreased in prostate cancer, however prostatitis or hemorrhage may also lead to decreased citrate levels on MRSI [90]. Metastatic poorly differentiated prostate cancer may show very low citrate levels [91, 92].
Prostate cancer cells have higher cell membrane surface area per cellular volume. High cellular tumor proliferation is associated with high turnover of cell membrane. Therefore, choline level, a by-product of phospholipid component of cell membrane, is increased on prostate cancer spectroscopy. In addition to each individual metabolite value in MRSI, choline-to-citrate and (choline + creatine)/citrate ratios are also representative of increased cell membrane turnover and malignant tissue and have been used as biomarkers for differentiating cancer from benign tissue.
Detection of Nodal Involvement
Currently, extended pelvic lymph node dissection (ePLND) is the gold standard for accurate detection of microscopic invasion of lymph nodes [93]. Imaging criteria for detection of LNM using CT and MRI include macroscopic enlargement of lymph nodes (size >10 mm for the short axis of an oval node or above 8 mm for the diameter of a round node). A meta-analysis comparing the performance of CT with conventional MRI showed equally poor performance with sensitivity of about 40 % and specificity of about 80 % [94]. There is no suitable size threshold to accurately detect LNM. However, the level of LNM differentiates the categorization as N or M stage, where the LNM above the level of common iliac vessels bifurcation is defined as M stage [95].
As a functional parameter, DWI can potentially be used to distinguish malignant from benign lymph node enlargement. Malignant lymph nodes typically exhibit restricted diffusion, owing to higher cellularity, enlarged hyperchromatic nuclei, and abundant macromolecular protein. In a study by Eiber et al. [96] the performance of DWI in conjunction with T2WI was investigated to detect LNM in patients with prostate cancer. Some patients had histological evidence of LNM through extended PLND and others were evaluated on clinical follow-up. It was shown that ADC values were significantly lower in malignant nodes compared with benign nodes in both subgroups. Authors identified that an ADC cut-off value of 1.30 × 10−3 could distinguish malignant from benign nodes better than short axis size cut-off of 9 mm; sensitivity 86 vs 82 %, and specificity 85.3 vs 54.4 %, respectively. However, DWI is subject to some limitations such as frequent artifacts and limited spatial resolution. Moreover, heterogeneous cohorts and lack of ePLND as gold standard in some patients in the reported studies make the interpretation of performance of DWI challenging.
Further advances have been made by introduction of lymphotropic MR contrast agent and several studies reported results from MR lymphography (MRL). Ultrasmall superparamagnetic iron oxide (USPIO) particles are virus-sized particles which are being accumulated in mononuclear phagocytic system. Therefore, they diffusely accumulate within normal lymph nodes. The normal uptake of USPIO by the macrophages exhibits a homogenously low SI in the normal lymph nodes. In LNM, due to tumor deposits in the lymph nodes instead of expected SI loss there is focal or diffuse increase in signal signifying replacement of the normal tissue by cancer. Early studies demonstrated that MRL was much more sensitive and specific than conventional MRI at 1.5 T to detect LNM (90.5 vs 35.4 %, and 97.8 vs 90.4 %, respectively). The difference was even higher for small-sized lymph nodes [97]. In another study, the performance of MRL at 3.0 T to detect LNM in patients with prostate and/or bladder cancer with no enlarged lymph nodes on previous CT or MRI was investigated. It was shown that MRL using quantification of signal-to-noise ratio (SNR) reduction could improve detection of LNM in normal-sized pelvic lymph nodes [98]. It has also been shown that MRL can detect LNM better than MDCT. In a study by Heesakkers et al. [99], it was reported that MRL could detect LNM with a higher sensitivity than MDCT (82 vs 34 %) in patients with intermediate or high risk of LNM (risk of >5 % according to nomograms). However, MDCT was slightly more specific than MRL (97 vs 93 %).
A combination of USPIO-enhanced MRI and DW-MRI was a promising method to detect LNM in normal-sized lymph nodes in a study by Thoeny et al. [100], who reported that this method was fast and reliable for nodal staging in patients with prostate and/or bladder cancer. The use of USPIO has been limited to investigational studies and has only been approved in some European countries, and to date has not been approved by the health authorities in the U.S.A. The production of the contrast agent has been discontinued and future clinical applications are unknown at this time.
Detection of Distant Metastasis
MRI can detect early bone marrow changes before osteoblastic changes appear in the bone marrow. MR appearance of bone metastases are signal loss which is in contrast to the high signal of surrounding marrow with fat on T1WI. The T2WI with fat suppression or short tau inversion recovery (STIR) are helpful to better visualize the bone metastases [22]. In a study by Lecouvet et al. [23] it was shown that MRI of discrepant or equivocal sites of bone lesions improved the bone metastases detection compared with BS combined with TXR, sensitivity 82 vs 63 % and specificity 100 vs 84 %, respectively. Figure 4.5 illustrates appearance of small bone metastasis on MRI, CT, and BS, and how the equivocal lesion on BS can be confirmed as benign by cross-sectional imaging.
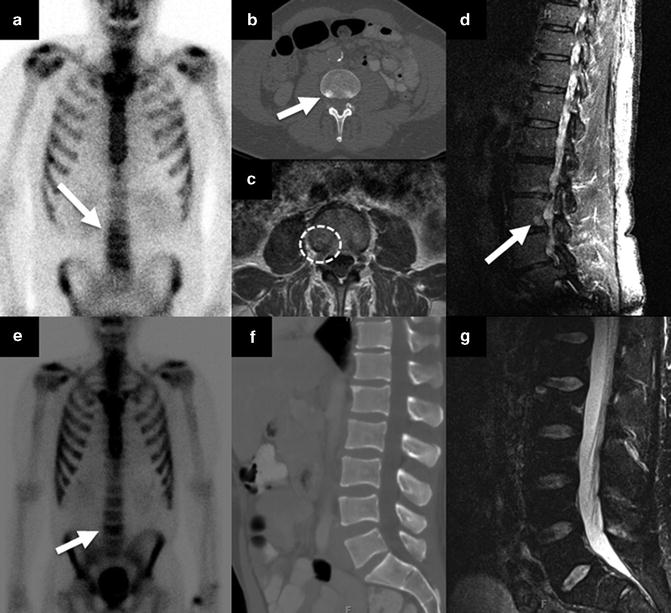

Fig. 4.5




Appearance of a bone metastasis and benign lesion on cross-sectional images and BS. A 67-year-old man with biopsy Gleason score 5 + 4, BS showed mild uptake on the right at the L3 vertebral body level (a), CT showed 1 cm rounded sclerotic focus on the right side of the L3 vertebral body (b), and confirmatory findings on MRI identified metastasis with focal signal loss combined with peripheral rim enhancement on T1WI (c) and high signal lesion on STIR sequence (d). A 53-year-old man with biopsy Gleason score 4 + 5, BS showed focal increased tracer uptake at L4 vertebral body level (e), CT revealed no focal lesion (f) with only minimal degenerative changes and likely lordotic strain responsible for the increased radiotracer uptake on E, as on MRI there was no abnormal signal on STIR sequence (g)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree