© Springer International Publishing Switzerland 2017
Francesco Bertoldo, Francesco Boccardo, Emilio Bombardieri, Laura Evangelista and Riccardo Valdagni (eds.)Bone Metastases from Prostate Cancer 10.1007/978-3-319-42327-2_1717. New Frontiers in Treatment
(1)
Medical Oncology, Department of Oncology Azienda USL Toscana Sud-Est, Istituto Toscano Tumori (ITT), Ospedale San Donato, Arezzo, Italy
In the United States, prostate cancer is the most common cancer in men, with an estimated 220,000 cases diagnosed in 2015 [1]. With a percentage of involvement of more than 80 %, the bone represents the preferential site of metastases for this disease. As a consequence, patients experiencing advanced stage castration-resistant prostate cancer (CRPC) are at increased risk of developing skeletal-related events, including pathologic fractures and spinal cord compression [2].
Despite recent important therapeutic advances in the management of CRPC, there is a continuous medical need to develop further treatment options to overcome the mechanisms of resistance of surviving prostate cancer cells, such as the splice variants of the androgen receptor (AR). All the possible new agents, or combinations, with efficacy data in the area of prostate cancer have been analyzed. Data are presented according to the mechanism of action of the single agents.
17.1 Vascular Endothelial Growth Factor (VEGF) Targeting Therapies
In prostate cancer, the VEGF signaling pathway seems to be para-physiological for disease progression: higher levels of VEGF receptor (VEGFR)-2 are observed in high-grade prostate cancer, while patients with metastatic prostate cancer have higher serum VEGF levels and levels of urine and serum VEGF seem to relate with overall survival (OS), in subjects with metastatic CRPC (mCRPC).
VEGFRs are also expressed in human osteoblasts and osteoclasts with the VEGF pathway involved in mechanisms regulating cell migration and survival [3–5]. Moreover, VEGF treatment inhibits the apoptosis of human osteoblasts by increased expression of the Bcl-2, an anti-apoptotic protein, as demonstrated in vitro [5].
All these findings suggest an important role for the VEGF signaling pathway in the processes of prostate cancer progression and bone metastasis.
Several agents targeting angiogenesis have been evaluated in phase III clinical trials in CRPC, but no one demonstrated a clinical benefit in men with CRPC.
17.1.1 Bevacizumab
Bevacizumab is a recombinant, humanized monoclonal antibody blocking VEGF activity. In CALGB 90006, a phase II study, 79 patients with chemotherapy-naïve metastatic CRPC received bevacizumab 15 mg/kg combined with docetaxel and estramustine. The progression-free survival (PFS) and median OS were 8 and 24 months, respectively. The observed improvement in OS led to plan a phase III study despite this study did not meet its primary endpoint of PFS [6].
The phase III, double-blind, placebo-controlled study, CALGB 90401, randomized 1050 chemotherapy-naïve mCRPC patients to docetaxel (75 mg/m2 every 3 weeks) with prednisone (5 mg BID) and either bevacizumab (15 mg/kg IV every 3 weeks) or placebo [7]. The primary endpoint of this trial was OS, while PFS, objective response (OR), and 50 % decline in PSA were secondary endpoints. Any statistically significant difference in OS was observed (22.6 months in the bevacizumab group vs. 21.5 months in control group; p = 0.181) despite an observed improvement in PFS and ORR in the experimental group. Moreover, the addition of bevacizumab was associated with greater treatment-related toxicities (grade ≥3 neutropenia, fatigue, leukopenia, hypertension, gastrointestinal bleeding, and perforation) [7]. As a comment, the OS time of the control group observed in this trial was longer than what reported in other studies (21.5 months vs. 19.2 months observed in TAX 327 study), raising doubts that the study may have been underpowered. Moreover, this trial was not designed to evaluate the role of maintenance of bevacizumab beyond disease progression, which seems to confer a clinical benefit in several types of cancer.
17.1.2 Sunitinib
Sunitinib is an oral multi-tyrosine kinase inhibitor (TKI) of VEGFR-2, PDGFR, FLT-3, and KIT, with a demonstrated activity in two previous phase II studies in patients with mCRPC who failed a previous docetaxel chemotherapy [8, 9].
A randomized, multicenter phase III trial enrolled a total of 873 subjects with progressive mCRPC, after docetaxel chemotherapy. Patients were randomized to sunitinib (37.5 mg daily) or placebo, in a 2:1 ratio. The primary endpoint was OS, with PFS as a secondary endpoint.
While the median OS time was similar in both groups (13.1 vs. 12.8 months, respectively; HR 1.03; 95 % CI 0.80–1.32; p = 0.5813), PFS was significantly longer in the experimental arm (5.6 vs. 4.1 months; p < 0.001) [10]. The study was stopped on recommendations of the data monitoring committee, after the results of a second interim analysis showing that it was unlikely for the study to meet its primary endpoint.
17.1.3 Lenalidomide
Lenalidomide is an oral immunomodulatory agent which inhibits VEGF signaling and angiogenesis [11]. The MAINSAIL phase III study randomized 1059 chemotherapy-naïve patients with mCRPC to docetaxel (75 mg/m2, once every 21 days) with prednisone (5 mg BID) and either lenalidomide (25 mg daily, days 1–14) or placebo. Also this study was discontinued on the recommendations of the Data Monitoring Committee, as it was unlikely to meet its primary endpoint (OS). Moreover, patients randomized to experimental arm had higher rates of febrile neutropenia and other non-hematological toxicities [12].
In a phase II trial in patients with mCRPC, a dual anti-angiogenic treatment with bevacizumab and thalidomide (another oral immunomodulatory agent) was also evaluated in combination with docetaxel. The median OS time (25.9 months) was significantly longer in the experimental arm, but this combination approach resulted also too toxic [13].
17.1.4 Aflibercept
Aflibercept is a recombinant fusion protein consisting of extracellular domains of VEGFR fused to the Fc of a human IgG1 antibody. In the VENICE study, 1224 chemotherapy-naïve patients with metastatic CRPC have been enrolled to receive docetaxel (75 mg/m2 IV every 3 weeks) and prednisone (10 mg daily) plus aflibercept (6 mg/kg IV, every 3 weeks) or placebo.
No difference in OS was observed between the treatment arms (22.1 months in aflibercept arm vs. 21.2 months in placebo arm, p = 0.38), while a statistically significant increase in the number of side effects was reported in the aflibercept arm [14].
17.1.5 Tasquinimod
Tasquinimod is an oral agent with anti-angiogenic activity but an unknown mechanism of action [15]. In a double-blind, placebo-controlled phase II study, 201 patients with mCRPC were randomized in a 2:1 ratio to either tasquinimod or placebo [15]. After an initial double-blind treatment (maximum of 6 months), asymptomatic subjects in the placebo group were allowed to switch to open-label tasquinimod.
The primary endpoint was the proportion of patients who were progression-free at 6 months by RECIST and Prostate Cancer Working Group 2 criteria. This endpoint was superior in the tasquinimod group over placebo (69 % of patients vs. 37 %, p < 0.001); also median PFS was longer in the tasquinimod arm over placebo (7.6 vs. 3.3 months, p = 0.0042). Of interest, subgroup analyses suggested a clinically relevant impact in PFS for tasquinimod, especially for those with bone metastases (8.8 vs. 3.4 months; p = 0.019). Grade 3–4 treatment-related toxicities were more common in the experimental arm (40 % vs. 10 %) [15].
Based on the phase II trial results, a phase III double-blind study was conducted in asymptomatic to mildly symptomatic CRPC patients with bone metastases. In this trial, a total of 1,200 subjects were randomized to tasquinimod or placebo. The primary endpoint was PFS, but the study was powered to detect an improvement in OS as a secondary endpoint. No improvement in OS was observed with tasquinimod (HR = 1.09; CI 95 %, 0.94–1.28), with reasons of this negative result not clear yet [16].
17.2 MET-Targeting Therapies
The HGF/MET pathway plays an important role in various human malignancies, including prostate cancer. A correlation was shown between higher levels of MET expression and higher grade of prostate cancer. Moreover, an increased expression of MET seems to relate with prostate cancer metastasis and the emergence of CRPC [17, 18]. Similarly to VEGF, MET signaling pathway seems also important for osteoblasts and osteoclasts. In prostate cancer, bone metastases are more likely to express MET than soft tissue and lymph node metastases [18].
Thus, an increased expression of MET seems to have an important role in bone metastasis from prostate cancer. Phase two studies specifically enrolling patients with metastatic prostate cancer are ongoing with two MET-targeting agents in development.
17.2.1 Rilotumumab
Rilotumumab (AMG102) is a human monoclonal antibody blocking the binding of HGF to MET. In a recent multicenter, double-blind, phase II study, 142 mCRPC patients progressive after taxane chemotherapy have been randomized (1:1:1), to mitoxantrone (12 mg/m2, every 21 days) and prednisone (5 mg BID) plus rilotumumab at 15 mg/kg or 7.5 mg/kg or placebo.
Median OS (primary endpoint) was similar among the groups (13.4 vs. 11.6 vs. 11.1 months, respectively). Any difference in PFS or PSA response was also observed among treatment arms [19].
17.2.2 Tivantinib
Tivantinib (ARQ 197) is an oral putative non-ATP competitive inhibitor of the c-MET receptor tyrosine kinase. A phase II study randomized 80 men with chemotherapy-naïve, minimally symptomatic or asymptomatic mCRPC to either tivantinib 360 mg PO BID or placebo. PFS was the primary endpoint of the study. Patients in the tivantinib group had a significantly better PFS in comparison with those in placebo group (medians, 5.6 months vs. 3.8 months, respectively; HR = 0.53, 95 % CI: 0.32–0.89; p = 0.015) and a favorable toxicity profile [20].
Other agents targeting the MET pathway are in earlier phases of clinical development, including onartuzumab (MetMab), TAK-701, ficlatuzumab, crizotinib, and JNJ-38877605.
17.3 Dual Inhibition of MET and VEGFR Signaling: Clinical Evidence
MET and VEGFR signaling pathways play an important role in the promotion of tumor cell growth, angiogenesis, invasion, and metastasis and in bone turnover in multiple tumor types including prostate cancer, especially in mCRPC cases with bone metastases.
Resistance to VEGF-targeted therapies may arise from the upregulation of alternative pro-angiogenetic and pro-invasive signaling pathways, including the MET pathway.
Considering the molecular pathophysiology of advanced CRPC, there was a strong rationale for the evaluation of cabozantinib, an orally TKI with potent activity against MET and VEGFR2 in this disease.
In addition, other agents targeting both MET and VEGFR signaling pathways are in development, including foretinib (GSK1363089), golvatinib (E7050), GSK1363089 (XL880), and MGCD265.
17.3.1 Cabozantinib
Cabozantinib is a novel, oral, multiple receptor tyrosine kinase inhibitor with an activity against MET and VEGFR2, as well as RET, KIT, AXL, and FLT [21]. In the initial clinical studies, cabozantinib demonstrated promising results in mCRPC and other malignancies.
After the initial phase I trial evidencing tumor responses in multiple malignancies, a randomized phase II discontinuation study (cabozantinib vs. placebo) was planned.
In the CRPC group of this phase II study, the 87 % of the enrolled patients had bone metastases and 43 % were pretreated with docetaxel.
Cabozantinib was administered at a daily dose of 100 mg during a 12-week lead-in stage. This dosage was associated with frequent side effects, leading to dose reductions in 51 % of subjects by week 12, with 16 % discontinuing therapy due to toxicity prior to week 12. The most common grade 3 toxicities were fatigue (16 %), hand-foot syndrome (6 %), and hypertension (6 %) [22]. A PFS prolongation was observed in the cabozantinib group compared with placebo (23.9 weeks vs. 5.9 weeks, respectively).
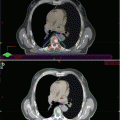
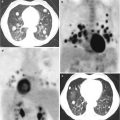
In addition, cabozantinib showed a partial/complete response in 99mTc-MDP bone scans in 56 and 19 % of patients, respectively. Among the cases with a present baseline pain, 64 % reported a decrease in pain intensity, with a third of them stopped narcotic pain medication.
Moreover, in the 40 % of treated patients, a discordance was noted between PSA and bone scan response. The high rates of bone scan improvements and the relevant clinical benefit led to a couple of phase III, randomized, double-blind, controlled trials (COMET-1 and COMET-2).
The phase III study COMET-1 [23] randomized 1028 mCRPC patients pretreated with docetaxel, abiraterone, and/or enzalutamide, to receive either cabozantinib (daily dose of 60 mg) or prednisone (10 mg/day) in a 2:1 ratio. Compared to prednisone, cabozantinib improved PFS (5.5 months for cabozantinib vs. 2.8 for prednisone (p < 0.001)) and bone scan response (41 % for cabozantinib group vs. 3 % for control group (p < 0.001)) but not significantly increased OS (11 vs. 9.8 months, p = 0.212)).
Disappointing negative results derived also from the COMET-2 study [24], where cabozantinib was compared with mitoxantrone and prednisone in 119 subjects with symptomatic disease. The primary endpoint, of durable pain response at week 6 (confirmed at week 12) without an increase in narcotic medication, was not achieved in this population with a pain response rates of only 15 % in the cabozantinib arm compared to 17 % in control group (p = 0.773).
17.4 Dual Androgen Synthesis and Signaling Inhibitor
17.4.1 Galeterone (TOK-001)
Galeterone (TOK-001) is a multifunction oral steroid analog that concomitantly: (1) decreases androgen biosynthesis by inhibiting the enzyme CYP17 (controlling androgen production in the adrenals, testes, and prostate), (2) decreases AR signaling by binding to the AR as a competitive inhibitor of testosterone, and (3) reduces the AR expression in prostate cancer cells by increasing the AR protein degradation and therefore diminishing the cell ability to respond to low levels of androgenic growth signals [25].
More recently, galeterone has been shown to downregulate the levels of constitutively active AR splice variants [26]. As known, these AR variants are upregulated in CRPC cells that have become resistant to CYP17 inhibitors and/or antiandrogens. Galeterone could be effective in CRPC cells expressing AR splice variants such as AR-V7 [27].
In the ARMOR phase I study of chemo-naïve men with CRPC, galeterone (TOK-001) was well tolerated and demonstrated clinical activity. Of 49 patients, 22 % demonstrated a >50 % PSA decline and an additional 26 % had PSA declines of 30–50 % [28].
Based on these preliminary results, a phase II study (ARMOR-2) was started in treatment-naïve cases. The interim data, in nonmetastatic/metastatic treatment-naïve CRPC receiving galeterone (2225 mg/day), showed a maximal reduction in PSA levels of at least 30 % (PSA30) in 8/11 (72.7 %) of the patients; 6/11 (54.5 %) of these patients showed a maximal PSA reduction of at least 50 % (PSA50). In M1 cohorts (N = 39), PSA30 and PSA50 were, respectively, 85 and 77 %. SD was observed in 72 % of patients with metastatic disease (13 out of 18) and PR in 17 % (3 out of 18).
Galeterone was well tolerated; the most common adverse events were fatigue, increased liver enzymes, gastrointestinal events, and pruritus. Most were mild or moderate in severity and required no action; there were no apparent mineralocorticoid excess (AME) events [29].
Galeterone shares similar MOA with enzalutamide (both drugs inhibit GABAA, which lowers the epileptogenic threshold). The nonclinical data (in vitro) support the clinical observations to date where galeterone has not been associated with an increased seizure risk.
Preliminary data from the ARMOR-2 study showed that six of seven treatment-naïve CRPC patients with high N-terminal AR expression and C-terminal AR loss had PSA reductions of at least 50 %, suggesting that galeterone may have activity in patients with AR splice variant, including AR-V7. According to these data, an open-label phase 3, randomized study (ARMOR-3) is ongoing to evaluate the efficacy and safety of galeterone, compared to enzalutamide, in patients with treatment-naïve metastatic (M1) castration-resistant prostate cancer (CRPC) expressing androgen receptor splice variant-7 mRNA (AR-V7).
17.5 Second-Generation Androgen Receptor Inhibitors
17.5.1 ARN-509
ARN-509 (also referred as JNJ–56021927) is an oral small-molecule, nonsteroidal potent and selective antagonist of the AR which acts by inhibiting the action of androgen, nuclear translocation of the AR, and DNA binding to androgen response elements. Unlike bicalutamide, it exhibits no significant agonist activity in AR-overexpressing prostate cancer cells [30]. Like enzalutamide, ARN-509 has been developed to overcome the therapeutic limits of the first-generation antiandrogens.
In a murine xenograft model of mCRPC, ARN-509 showed greater antitumor activity than enzalutamide, for a given dose and plasma concentration. Furthermore, ARN-509 achieved significantly lower steady-state brain levels in respect to enzalutamide, suggesting a lower seizurogenic potential [30].
The ARN-509-001 phase I/II trial enrolled 30 cases with mCRPC and reported a promising activity [31]. At 12 weeks, 42 % of patients displayed a ≥50 % PSA declines with a fluoro-dihydrotestosterone (FDHT)-PET imaging demonstrating an AR blockade at 4 weeks across multiple doses. JNJ-56021927 (ARN-509) was safe and exhibited linear pharmacokinetics.
The phase II evaluation showed a PSA response at 12 weeks of 91 % in therapy-naïve and 60 % in post-abiraterone acetate mCRPC patients and 89.5 % in nonmetastatic CRPC patients [32, 33]. The phase 3, randomized study ARN-509-003 (SPARTAN) of ADT+ARN-509 compared with ADT + placebo, in patients with high risk (defined as a PSA doubling time ≤10 months) nonmetastatic CRPC, has completed the accrual, and data are not available yet.
According to the distinct mechanism of action of ARN-509 and abiraterone acetate (AA inhibits androgen biosynthesis while ARN-509 targets the AR) and the absence of overlapping clinical toxicities, the combination of both drugs could theoretically delay the emergence of clinical resistance to either drug.
In xenograft models of CRPC, treatment with AA causes a marked suppression of tumor androgen levels which rely with an increased expression of the AR, ligand-independent AR splice variants, and inductions of steroidogenic genes including CYP-17-A1 [34]. The increased expression of several of these genes showed strong correlation with dihydrotestosterone (DHT) levels in recurrent tumors. These data suggest that resistance can potentially be targeted by using combinations with potent AR antagonists such as ARN-509. An ongoing, randomized, double-blinded, placebo-controlled study (56021927PCR3001) is designed to assess the efficacy and safety of ARN-509 in combination with AA + PDN compared with AA + PDN in patients with chemotherapy-naïve mCRPC and will explore mechanisms of resistance that may develop with treatment.
17.5.2 ODM-201
ODM-201 is a novel AR antagonist structurally distinct from all known antiandrogens. In vitro receptor binding studies show that ODM-201 and its major metabolite, ORM-15341, bind with high affinity to wild-type AR inhibitors [35]. In murine castration-resistant VCaP xenograft models, ODM-201 achieves an improved inhibition of tumor growth compared to enzalutamide [35]. ODM-201 also shows an inhibitory activity, without evidence of agonism, against several mutant ARs implicated in resistance to other second-generation AR inhibitors [35]. These include AR F876L, which causes antagonist-to-agonist switching with both enzalutamide and ARN-509 in preclinical models of prostate cancer and which has been identified in plasma DNA from patients with progressive CRPC treated with ARN-509 [35–37]. In contrast to other second-generation AR inhibitors, preclinical studies suggest that the penetrance of ODM-201 and ORM-15341 through the blood–brain barrier after oral administration is negligible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree