Neurosurgical Interventional Approaches to Pain
Andrew Mannes
Philip S. Kim
Russell R. Lonser
Interventional and neurosurgical procedures can be utilized to supplement pharmacologic and complementary approaches break to treat pain (see chapters 3 and 4). Pharmacologic therapies are described elsewhere in this text and includes principles of analgesic management using opioid agents and adjuvant medications. The primary indications for interventional techniques are for patients whose pain is either poorly responsive to systemic analgesic therapies or for those who suffer from intolerable side effects, in whom efforts to manage adverse effects are unsuccessful. Patients can experience severe dose-limiting side effects that prevent optimal titration to therapeutic levels. For example, systemic opioids can produce constipation, nausea, vomiting, or sedation.
Patient’s response to analgesic medicinal therapies has been best described in the cancer population. The oral administration of analgesics based on recommendations, including those outlined by the World Health Organization, has provided satisfactory relief to most patients. However, the poorly relieved pain experienced by 5 – 15% (1, 2, 3) of the approximately 500,000 patients who die each year from cancer represents a significant need for additional methods, including interventional and neurosurgical procedures, that can offer symptom relief.
Aside from optimizing pain control while minimizing side effects, interventional pain therapies can also enhance functional abilities and physical and psychological well-being, enhancing the patient’s quality of life (4). It has also been reported that better pain management utilizing interventional techniques may result in increased life. Further, reducing patient visits for symptom management could potentially reduce costs (5).
Initial Evaluation
For the interventionalist, it is important to understand the patient’s prognosis, associated comorbidities, and patient’s and family’s expectations. An initial evaluation for interventional pain therapies should ascertain the patient’s general medical condition along with the primary disease. A complete history is required, including a general medical, disease-specific (e.g., patients with oncologic disorders need to be thoroughly evaluated for possible local recurrence or new metastases), and pain histories. Specific pain history would include the following: quality of pain, pain intensity, alleviating and exacerbating factors, temporal characteristics, duration, and associated features (e.g., numbness, weakness, vasomotor changes). Psychosocial evaluation should assess the presence of psychological symptoms (e.g., anxiety, depression), and psychiatric disorders (e.g., major depression, delirium) should be similarly addressed. The nature and meaning of the presenting pain needs to be distinguished from anxiety and suffering affecting all aspects of one’s life. The ability to cope and the availability of psychosocial support systems need to be assessed and reinforced with proper health and social professionals. A final assessment should determine the patient’s expectation of therapeutic interventional options.
The physical examination includes a general medical examination, with emphasis on neurologic findings. Specific examination of the site of pain and surrounding anatomic regions are important. For example, if a patient has motor and sensory deficits in a particular region, neurolysis techniques become a more acceptable therapeutic option.
Appropriate selection of an intervention is based on therapeutic goals. If the presenting pain is expected to be transient (pain that will be alleviated by primary radiation therapy or chemotherapy, pain that is associated with the treatment of the primary disease, or pain that is)radiation therapy or chemotherapy, treatment of the primary disease, or pain that is associated with the therapy itself) then the intervention should likewise be reversible. However, if the pain is expected to be chronic, a technique that results in more permanent effects is indicated. Life expectancy must be considered when selecting an appropriate intervention. If the patient’s life expectancy is short, treatment strategies should strive to minimize the frequency and level of interventions and recovery time and should focus on optimizing a patient’s quality of life. A patient with a longer life expectancy may warrant more extensive and expensive interventions (i.e., implantable devices). Certain procedures may not be indicated for patients with longer life expectancy such as neuroablative procedures that are associated with permanent loss of function or a theoretical risk of developing deafferentation pain syndromes.
Therefore, once a definitive diagnosis has been made, a treatment plan should characterize the expected outcome, define contingencies, and plan for reassessment. Longitudinal monitoring of pain and response to interventional therapies is essential and allows implementation of additional options (e.g., complementary therapies, pharmacologic strategies, and behavioral and psychological approaches).
This chapter includes some of the frequently utilized procedures in the palliative care pain population (Table 7.1). Not all indications and contraindications are included and consultation with a pain practitioner should be considered before referring the patient for evaluation and treatment.
Table 7.1 Comparison of Interventional Pain Procedures | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Approaches to Interventions
Pharmacologic management of pain can be viewed as a continuum of indirect and direct drug delivery paradigms (4). Indirect drug delivery (i.e., systemic analgesia) refers to the administration of an analgesic into the bloodstream, which is then transported to the receptor site in neural tissue:
By systemic absorption
By formulation of depot for sustained and continuous release
Through the bloodstream
Direct drug delivery is the administration of an agent to the targeted neural tissue involved in nociception. By delivering directly to the nociceptive pathways, one can achieve a pronounced analgesic effect at a lower dose with fewer side effects. An example of this is comparing equianalgesic morphine doses in the intrathecal, epidural, and intravenous spaces (Table 7.2) (6).
Interventional pain therapies are usually minimally invasive techniques that can be divided into direct drug delivery, neuroablation, neural blockade, and neurostimulation. Direct drug delivery involves the administration of analgesics, usually opioids and local anesthetics, directly into nociceptive pathways. Other potential agents such as α2-agonists and calcium channel blockers can be administered. Neuroablation refers to direct chemical, thermal, or surgical destruction of nociceptive pathways. Neurostimulation or neuroaugmentation refers to the application of direct electrical stimulation to inhibit nociceptive transmission. Not all pain, however, can be adequately addressed using these techniques. In such cases, one can consider consultation with a neurosurgeon about surgical intervention.
Direct Drug Delivery
Neuraxial direct drug delivery involves accessing the epidural or subarachnoid (intrathecal) space by a needle or the placement of a continuous infusion system. In general, neuraxial infusion should be considered when severe pain cannot be controlled with systemic drugs and/or because of dose-limiting toxicities. Neuraxial infusions can also be considered when there is an immediate need for using various nonopioid analgesics. Specifically, local anesthetics can have a profound analgesic effect on many intractable opioid-unresponsive pain conditions. Although it is possible to give local anesthetic systemically, higher local concentrations can be achieved, resulting in profound neural blockade through direct drug delivery.
Table 7.2 Equianalgesic Morphine Conversions Among Routes of Administration | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Neuraxial delivery systems have two components: an intraspinal or epidural catheter and a delivery mode (e.g., bolus dosing, syringe pump, internal port, or internal or external pump). There are basically five types of neuraxial drug delivery systems, and familiarization with these systems allows the clinician to understand the respective advantages and disadvantages of each (7, 8).
The simplest, least expensive, and least invasive option, a percutaneous catheter, is typically made of nylon, polyurethane, or polyamide and can be wire reinforced. These catheters are routinely placed in surgical and obstetric patients to manage operative and postoperative pain and are designed for short-term use (<1 week). But a catheter may be maintained for longer periods without problems and may suffice for the duration of the patient’s life. If there is a complication, these catheters can be discontinued by removing the dressing and withdrawing the catheter. However, these catheters can cause localized tissue reaction at the site of insertion, can migrate, and are susceptible to accidental displacement.
The next type of drug delivery system uses the same type of catheter as that mentioned in the preceding text, but it is tunneled subcutaneously to decrease the incidence of migration. Placement can be performed in a clinic and requires a small incision with multiple needle insertions. Tunneling the catheter is better suited for the outpatient or the home-bound patient.
Implanted catheters with subcutaneous injection site are technologically more advanced and require a minor surgical procedure, resulting in higher costs (for placement). Sterile preparation and the use of fluoroscopy are essential. These systems can be placed in the epidural or intrathecal space. There are two basic designs: exteriorized or completely internalized injection port. In the first design, the proximal catheter is tunneled from the exit site in the back and exteriorized usually along the midaxillary line. This catheter can include an antimicrobial cuff that reduces both infection and catheter migration. In the second design, the port is supported by bone, usually a rib, so as to facilitate needle insertion. It can be used for intermittent bolus dosing or accessed for continuous infusions.
A totally implanted catheter with implanted reservoir and manual pump is being developed by Medtronics, Inc. (Minneapolis, MN). The Algomed implantable patient-activated device is not yet commercially available. It is composed of an implanted reservoir with a manual pump. This design allows patient-controlled analgesia by pressing the activation valve and pumping chamber, providing a bolus of medication. Because the entire device is implanted, the reservoir is refilled by inserting a needle into a subcutaneous port in the control pad.
A totally implanted catheter with implanted infusion pump is available in two basic designs. The simpler design is a constant fixed infusion pump in which the dose can be adjusted by a clinician changing the concentration (Johnson & Johnson). The second type includes a programmable, peristaltic infusion pump with a drug reservoir, an electronic module, and an antenna allowing reprogramming of drug flow rates (Medtronic Inc., Minneapolis, MN). The clinician controls the pump through an external programmer head (such as a pacemaker) to alter the dose, give single doses, or change the continuous infusion rate.
Recently, Medtronic has received U.S. Food and Drug Administration (FDA) approval for a patient activation device that will allow the patient to receive a medical direct bolus of medication when the device is activated.
Typically, a percutaneous test catheterization of the epidural or intrathecal space would be performed to assess the efficacy and starting doses of medication before implanting a permanent delivery system. There are several approaches to trial the drugs, including bolus dosing, by accessing the intrathecal space with a spinal needle, or by placement of a catheter and continuous infusion— either in the epidural or intrathecal space. Ideally, the method utilized for clinical assessment would best emulate the intended route (e.g., a trial with a continuous intrathecal catheter for evaluating future implantable pump placement). Although the complication rate is low, implantable devices can have problems with catheter failure, infection, seroma, wound dehiscence, and catheter tip fibroma formation (reported with high-concentration morphine) (9). They also require health care provider visits for routine refills and adjustment of dosing.
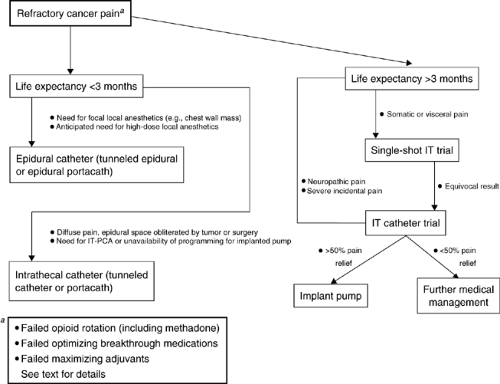 Figure 7.1. A decision-making algorithm for patients with refractory cancer-related pain. IT-PCA, intrathecal patient-controlled analgesia. |
The selection of the appropriate neuraxial drug(s) and delivery system for an individual patient is based on several considerations (7, 10):
Patient life expectancy
Economics and cost-effectiveness
Choice of epidural versus subarachnoid route of administration
Patient life expectancy and duration of need is difficult to predict. The more sophisticated implantable systems are expensive devices that require a trial catheter, adjustments of medications, and a surgical procedure for placement. One study by Bedder et. al. suggest that an implanted pump system is a more viable financial alternative compared to other drug delivery systems for a period over 3 months (11). The less sophisticated percutaneous and tunneled catheters are best suited for patients with a limited life expectancy of <1 month. Both epidural and subarachnoid drug delivery can be equally effective. The duration of therapy will usually predict the type of infusion system selected. Catheter obstruction, fibrosis, and loss of analgesic efficacy are well described in long-term epidural drug systems (7). Therefore, intrathecal drug delivery systems are best suited for a protracted duration of therapy (<3 months). A decision-making algorithm for using neuraxial analgesia is shown in Figure 7.1 (12).
Multiple pharmacologic preparations have been administered through the neuraxial drug delivery systems. The gold standard is morphine, which is widely used and successful. When intrathecal morphine provides inadequate relief, other opioids, such as hydromorphone, meperidine, methadone, fentanyl, and sufentanil, have been used. As tolerance develops, one might switch opioids and or use them in combination with coanalgesics, which include local anesthetics (e.g., tetracaine, bupivacaine), α2– agonists (e.g., clonidine), and GABA B agonists (baclofen). Most recently, ziconotide, a calcium channel blocker that is the synthetic equivalent of a peptide produced by a snail, has been approved for use in intrathecal pumps (13). Drug selection is based on the patient’s pain symptoms using clinical strategies which have been developed (e.g., the 2003 Polyanalgesic Consensus Conference) (14). The guidelines and algorithms were developed by an expert panel, evaluating existing literature and algorithms for various intrathecal drugs. The optimal drug dosage, concentration, and issues related to compounding of drugs has been reviewed.
Complications from neuraxial catheter and pump placement may result from anatomic changes, infection, fluid collection, catheter migration, or device failure. Patients with suspected block of the subarachnoid circulation due to tumor extension or subarachnoid hemorrhage/arachnoiditis may have a poor response to the delivery of intrathecal analgesia. Evidence of an obstruction should be sought using magnetic resonance imaging (MRI) or myelography to determine the level of obstruction. Retesting the efficacy of analgesia by placing the injectant proximal to the obstruction may yield improved analgesic response. Migration or fracture of the catheter should be suspected if the patient reports sudden changes in pain relief or if a fluid collection is seen at the insertion site. Percutaneously placed catheters can be bolused with a test dose of a local anesthetic to assess function. Myelography should be performed with implantable catheters or pumps (through a side port) to determine catheter placement and function when displacement or catheter rupture is suspected.
An infection of the site does not always necessitate immediate removal of a catheter. Superficial infections may only require a course of antibiotics. However, persistent or progressive tissue infection or Central Nervous System (CNS) involvement necessitates immediate removal of the catheter and/or pump.
A growing body of case reports and studies supports the benefits of direct drug (intrathecal) delivery systems. In a study of 202 patients experiencing refractory cancer pain who were randomized to receive either an implantable drug delivery system or comprehensive medical management (15), the patients receiving implantable intrathecal pump had reported successful pain control with a reduction in common drug toxicities such as fatigue and diminished level of consciousness. Overall, there was an improvement in the quality of life measures and survival over the 6 months in the patients receiving implantable intrathecal pump.
Peripheral Nerve/Plexus Drug Delivery
Blockade of peripheral nerves and neural plexi is commonly performed to provide regional anesthesia and analgesia to patients undergoing surgical procedures (15, 16, 17, 18). Modified constant infusion systems typically deliver local anesthetics directly to peripheral nerves and neural plexi in patients with inadequate analgesia or intolerable toxicities from systemic medications. Specific localized pain syndromes related to a mononeuropathy, plexopathy, and peripheral neuropathy may benefit from peripheral nerve infusion.
Continuous neural blockade of the brachial plexus is common for postoperative pain. A technique of placing a catheter along the brachial plexus and self-contained infusion system has been described (16, 17). A case report describes the successful 2-week management of pain from Pancoast’s syndrome with a brachial plexus infusion system using local anesthetics (19). Other potential areas where neural infusion could be performed include the lumbosacral plexus, paravertebral and selected peripheral nerves, and sympathetic chain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree