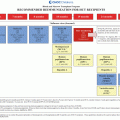
Fig. 16.1
Interactions related to functional status of brain tumor survivors based on Dennis’ multifactorial model [26]
A number of known primary and secondary factors have been demonstrated to impact neurocognitive outcomes in children with tumors of the CNS. Primary factors include treatment dynamics (surgery, chemotherapy, radiotherapy) and disease or tumor characteristics. Secondary factors include individual patient characteristics such as age and gender, elapsed time since treatment, and neurological sequelae (e.g., hydrocephalus, seizures).
16.2.3 Primary Factors
Surgery. Surgical resection of tumors is not typically discussed as one the most prominent factors associated with functional outcomes, and the lack of assessment of pre-surgical functioning is limiting. Nonetheless, there has been some suggestion of disruptions in verbal memory, visuospatial skills, and motor functions [27] in a group of children treated with surgery exclusively. Approximately 25–33 % of patients undergoing resection of posterior fossa tumors (primarily medulloblastoma) suffer cerebellar mutism syndrome (CMS), a complication considered to be related to surgical intervention [28]. Transient features of this syndrome include acute mutism, irritability/agitation, ataxia, and hypotonia that typically emerges 2–3 days after resection and can last from days to months. The length of these symptoms relates to outcome, such that longer time to resolution relates to greater long-term impairments. Beyond the acute symptoms of CMS, various neurocognitive deficits persist, including executive dysfunction, particularly related to attention, cognitive and behavioral flexibility, strategic planning, and initiation [28]. While the cause of this complication in some pediatric patients remains elusive, young patients who developed CMS following surgery for medulloblastoma were more likely to exhibit brainstem and cerebellar medullary angle involvement pre-operatively [29]. There is also evidence of significantly greater atrophy of the total cerebellum, vermis, and brainstem in the children with CMS compared to those without CMS 1 year post-operatively. A difference of 15 IQ points between the groups on average was evidenced, and 60 % of the CMS group measured in the impaired range, compared with only 14 % in the non-CMS group [29]. These findings hint at a pathologic mechanism involving disruption of the dentatothalamocortical outflow pathway, which may relate to the more chronic functional impairments in CMS, namely the neurocognitive and behavioral sequelae. This is a reasonable hypothesis given the recent understanding of the relationship of the cerebellum to higher order cognitive functions, including executive functions, and emotional regulation [30].
Chemotherapy. Assessing the role of chemotherapy exclusively in pediatric brain tumor patients is difficult, and occluded by the involvement of multiple simultaneous treatment approaches (e.g., multi-agent chemotherapy, radiotherapy). Nonetheless, the Head Start II study suggested that within the first 2 years post-treatment, young children with malignant brain tumors treated with chemotherapy and autologous hematopoietic cell transplantation (AuHCT) were spared some of the neurocognitive consequences associated with early radiation therapy [31]. Similarly, children with medulloblastoma treated with chemotherapy alone were shown to function significantly better than children who received radiation, although still significantly worse than normal controls [32].
One study attempting to evaluate the effects of chemotherapy regimens in patients with medulloblastoma revealed significant neurocognitive susceptibility in patients treated with intrathecal methotrexate (MTX) compared to those treated with intravenous MTX or controls [33] as they performed significantly worse on all neuropsychological measures. However, another small study of children with chiasmatic-hypothalamic tumors reported no significant change in IQ over time related to MTX delivery methods [34].
The timing of chemotherapy, specifically MTX, has also been shown to relate to outcome, and the effect is pronounced in females, as well as in the younger cohort. Specifically, the greatest functional impairments are observed in children who received concurrent MTX and radiation therapy versus those who received MTX prior to radiation [35, 36]. Further, IT MTX is more commonly associated with the development of leukoencephalopathy, which also impacts neurocognitive functioning [37].
Radiation. Radiation therapy (RT) has been demonstrated to be a major factor in neurocognitive outcomes in pediatric neuro-oncology and treatment approaches have been altered over the past decade in an attempt to minimize these sequelae, while still contributing to survival. Specifically, reduced doses and volume considerations (focal or conformal approaches) have been the focus of research.
Establishing the lowest curative dose through reduced-dose craniospinal and whole brain RT has been a target of research [38, 39], as a clear dose-dependent relationship with cognitive functions in this population has been established [40–43]. Children receiving conventional craniospinal radiation dosing (55 Gy) more often have a high level of cognitive morbidity, and significant deterioration in global intellect over time, in some cases in excess of 25 IQ points [44–46]. About 23–50 % of survivors receiving high radiation doses demonstrate IQ’s less than 80 (≥1.5 SD below the mean) at follow-up [47, 48]. Additional areas of neurocognitive impairment have also been demonstrated to be related to higher radiation doses [49], including diminished processing speed [41, 43], attention, verbal reasoning and memory [43], visual-perception, language, and academic functioning [42, 50]. When radiation at higher doses (>45 Gy) involves total brain, supratentorial regions, and particularly the left temporal lobe, there is a higher susceptibility for greater neurocognitive declines [51, 52] including poorer intellectual outcomes [52].
Less radiation-related neurotoxicity has been demonstrated in reduced-dose RT studies, primarily related to global intellectual outcomes. However, even reduced doses carry some morbidity, and average IQ decrements of four points per year have been documented [53]. Comparisons made between craniospinal radiation doses on functional outcomes reveal greater neurotoxicity at 36 Gy, than 23.4 or 18 Gy. Patients treated with 36 Gy perform an average of 8–13 IQ points lower than those receiving 23.4 Gy [38, 40]. Douw’s study comparing low grade glioma patients with or without low-dose radiation therapy revealed significantly poorer executive functioning and processing speed in the radiated group, while core memory and motor functions did not appear to be affected [54]. Neuroanatomically, acceleration of white matter volume deterioration has also been linked to doses of 36 Gy even compared to 23.4 Gy [20].
Beyond differences in radiation doses comparisons between craniospinal RT or whole brain, and focal radiation reveal differential outcomes in intelligence and other neurocognitive domains, primarily with regard to severity. In contrast to the 25 point decline in IQ documented in the groups of patients requiring craniospinal RT/whole brain doses, an average 6–11 point IQ decline has been demonstrated in partial brain radiation [55]. Jalali and colleagues [51] reported a >10 % diminishment in IQ in a third of patients in their study, 2 years after focal radiation (primary diagnosis was craniopharyngioma). Compared to children receiving only focal posterior fossa radiation, craniospinal doses of 36 and 24 Gy resulted in 21 and 13 IQ point differences, respectively [40]. Other neurocognitive and functional domains are also impacted by focal approaches, including processing speed, attention, auditory and visual learning/memory, academic skills, adaptive functions, and behavior [55–57]. This again highlights that although the severity of neurocognitive impairments may be diminished with focal radiation, there remains a notable number of neurocognitive sequelae over time.
Several factors mediate the relationship between radiation and neurocognitive outcomes. Age has shown to account for more variance than radiation, and when combined, represent an additive effect on outcome [51]. These effects are particularly salient for those patients diagnosed and treated at younger ages [42], and it has been suggested that long-term IQ can be predicted by baseline intellect, radiation dose, and age at time of radiotherapy [38]. There is currently no conclusive evidence that reduced doses result in better outcomes in older children [58, 59].
Technological advances have resulted in the increased knowledge and availability of proton beam radiation therapy [60, 61]. Proton radiation allows for both higher doses of treatment to the tumor (compared to photons) and increased sparing of normal tissue [62]. This is possible as photon radiation delivers the greatest dose at the entry point, where proton radiation delivers its greatest dose at the target location. Significant structural sparing was identified when treating childhood ependymoma with proton-versus-conventional or conformal Intensity Modulated Radiation Therapy (IMRT) [63]. Similarly, proton treatment reduced the radiation dose to the temporal lobes and cochlea in treating medulloblastoma [64], which is important as the inadvertent involvement of healthy tissue even in the most advanced conformal techniques has been shown to relate to neurocognitive and functional deficits [65, 66]. As the hippocampus is known to be sensitive to radiation, and hippocampal injury is particularly associated with cognitive changes, reduced risk of insult to the hippocampus may likely preserve cognitive (especially memory) functions [62]. Laffonde and colleagues reported that in a cohort of craniopharyngioma patients receiving proton therapy between 1995 and 2007, evaluated approximately 4 years post-therapy, approximately 33 % were reported to have significant executive dysfunction [67]. While the potential benefits of proton beam radiation on neurocognitive outcomes has yet to be fully demonstrated, it is expected that minimizing exposure of healthy tissue to radiation may positively impact outcomes. This new technology may allow for radiation therapy in the youngest patients, potentially increasing survival rates, while limiting the disruption in neurocognitive development. Furthermore, proton therapy is posited to reduce the rate of secondary malignancies [62].
Molecular genetics is becoming an increasingly important focus of research in the area of long-term functional outcomes in children treated with radiation. There is some evidence showing that specific genetic polymorphisms (GSTM1 and GSTT1) are related to poorer neurological and neurocognitive outcomes in medulloblastoma survivors [68]. Patients with the most significant post-radiation sequelae have been shown to have greater than four single nucleotide polymorphisms (SNPs) in candidate genes [69]. Further, Svensson and colleagues documented crude differences in gene expression profiles between patients with and without severe adverse effects from radiotherapy over time [70].
Tumor-Related Factors. While tumor location has not been the primary target of research on neurocognitive outcomes in pediatrics, the studies that have been carried out have produced mixed results. Several studies have revealed evidence of particular susceptibility for tumors of the supratentorium, especially those involving the left hemisphere [48, 71]. However, other studies have failed to identify any differences in neurocognitive outcome between supratentorial and infratentorial tumors [27, 72, 73] or greater impairment with tumors of the posterior fossa [74, 75]. Patel and colleagues found greater neurocognitive and behavioral impairment in children with infratentorial tumors [76]. Attention, working memory, and language-based reading impairments were significantly more affected in the infratentorial group, even after controlling for the age at diagnosis. Nonetheless, both groups showed equivalent deficits in cognitive efficiency (processing speed), verbal learning/memory, and nonverbal intellectual functions. In addition, when assessing neurocognitive functions beyond global IQ in patients with cerebellar astrocytoma, specific impairments in processing speed, attention, executive functions, memory, and visuospatial skills were documented [72, 77]. Third ventricle tumors (e.g., craniopharyngioma, hypothalamic glioma) have also been documented to be susceptible to greater functional impairments based on tumor location [47, 48]. In one study of pre- and post-operative neurocognitive status, evidence of impairment prior to any treatment was found, and predictors of increased risk included severe pre-operative hydrocephalus, diagnosis of medulloblastoma, and brain stem infiltration [17].
Tumor Recurrence. Children who suffer tumor recurrence have not been well-researched, which may relate to lower survival rates in part. We know little about the differences between those who have recurred and those who have not, although there is some basic evidence to suggest that they are at higher risk for more significant functional disruptions than those in which the initial tumor was eradicated [78]. This is most likely related to the necessity for additional treatments, including repeat surgery, chemotherapy, and sometimes additional radiation [78].
16.2.4 Secondary Factors
Age and Time Since Treatment. A well-documented factor in outcome has been patient age at the time of diagnosis and treatment. The most devastating outcomes are observed in the youngest patients. Different cut-offs have been used, but in general, treatment initiated prior to the age of seven has been shown to have a negative impact on neurocognitive outcomes [9, 10, 52, 79, 80]. These findings have prompted changes in treatment approaches, most notably related to the initiation of radiotherapy in the very young (3 years and younger) [81]. Current approaches to treating the very young include delaying radiation as long as possible by initiating an intensive chemotherapy regimen [1, 82]. Sparing of long-term IQ changes has been demonstrated in patients treated with combined chemotherapy and reduced or no radiation compared to those treated with conventional radiation [50].
The time since diagnosis and treatment of brain tumors is negatively correlated with neurocognitive outcomes [9]. Early research on global cognitive development in this population revealed a diminishment of full scale IQ in the years following the end of treatment at a rate of 2–4 IQ points per year [7, 8, 10, 38, 83–86]. Studies have demonstrated more rapid decline in the first few years following treatment, followed by a leveling off over time [7, 43]. However, this trajectory is moderated by age, as those younger at the time of treatment take longer to reach a plateau [11]. In general, these declines continue for more than a decade following treatment [11, 12]. However, this is rarely related to a loss of previously acquired skills, rather the pattern exemplifies a lack of appropriately paced development over time relative to the normative population [11] (Fig. 16.2).
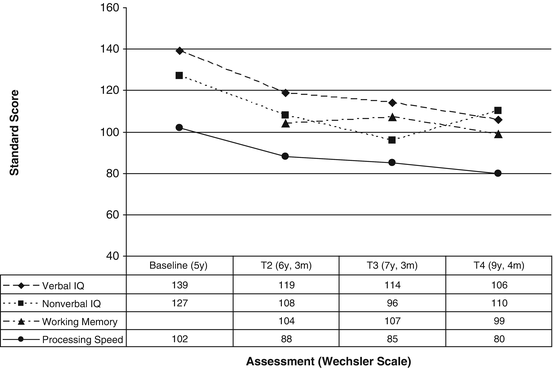

Fig. 16.2
Intellectual trajectory of medulloblastoma patient treated with craniospinal radiotherapy and chemotherapy following resection
Gender. Gender has also been demonstrated to be a factor in neurocognitive outcomes in some studies [87–90], with females being particularly susceptible to the effects of treatment for brain tumors. However, this potential difference has not prompted gender-specific treatment approaches to date.
Hydrocephalus. Hydrocephalus is a complicating factor in outcomes for pediatric brain tumor survivors. Children who have untreated hydrocephalus or require permanent shunt placement are at higher risk for long-term neurocognitive deficits [91]. The presence of hydrocephalus at the time of diagnosis results in significantly lower IQ at baseline, and the most opportunity for improvement following treatment [80], suggesting a reversibility of the effects of hydrocephalus when appropriately addressed. However, there is evidence that compared to medulloblastoma patients without shunts, those with ventriculoperitoneal (VP) shunts exhibited significantly lower IQ, academic skills, and visuomotor functions [92].
Seizures. The presence of seizures has been documented to be another complicating factor in outcomes for pediatric brain tumor survivors. A significant proportion of children with seizures demonstrate global intellect significantly below expected levels [78]. Klein documented greater neurocognitive impairment in patients with low-grade glioma who required treatment with anti-epileptic drugs (AEDs) [93]. The presence of seizures and AEDs in patients with third ventricular tumors was also demonstrated to negatively impact verbal memory more severely than those not requiring medical treatment for seizures [16].
16.3 Education and Employment Outcomes
There are obviously many sequelae associated with treatment of CNS tumors in childhood, which can affect the survivor’s ability to transition successfully into adolescence and adulthood. This particular topic will be more fully covered in this manuscript, but a summary of issues as they related to CNS tumors will be reviewed here. The full extent of these relationships is yet to be fully determined. However, several researchers have begun to explore this topic, with one of the longest and most successful being the Childhood Cancer Survivor Study (CCSS), discussed elsewhere in this manuscript as well. Briefly, the CCSS includes a cohort of over 14,000 survivors of childhood cancer, including CNS tumors, who are followed over time with questionnaires assessing physical, neurocognitive, social-emotional, and socioeconomic outcomes. Research emerging from the parent study repeatedly highlights increased rate of impairment in brain tumor survivors, consistent with lab-based research presented above. Specifically, these studies repeatedly highlight that brain tumor survivors have increased rates of impairment compared to controls for attention, processing speed, working memory, and long-term memory (Task Efficiency and Memory subscales on the CCSS Neurocognitive Questionnaire—NCQ) [3, 94]. This risk of impairment was increased for those who received RT, required a VP shunt, suffered a stroke, or evidenced other neurological disturbances such as hearing or vision impairment or paralysis [3, 94]. Further, lower neurocognitive ratings have been associated with reduced educational attainment, employment, and income status [94].
A crucial step in achieving independence in adulthood is completion of secondary and post-secondary education. Compared to all other cancer survivors, brain tumor survivors are the least likely to attend college, and 11 % less likely to do so than their siblings [95, 96]. The CCSS study has documented that nearly a quarter of survivors (compared to 8 % of sibling controls) were reported to receive special education services [97]. Despite this increased use of supportive special education services, CNS tumor survivors were more likely to repeat a grade and have been shown to perform more poorly in Math, English, and Science courses than their peers [98]. Additionally, completion of high school and post-secondary education was less likely, and there were higher rates of unemployment and diminished income levels compared to controls [99].
As teens transition to adulthood, the measure of age appropriate development often includes employment. Survivors of childhood cancer have been shown to be twice as likely to be unemployed compared to healthy controls, especially later in life [100]. Results from a meta-analysis revealed that survivors of CNS cancers were five times more likely to be unemployed than controls, with an unemployment rate ranging from 25 to 50 % [100]. Within the general cancer survivorship population, younger age at diagnosis, radiation treatment, lower baseline IQ, female gender, physical health, including pain, neuromotor, and neurosensory impairments, were significant predictors of limited employment and ability to maintain independence [100].
A number of non-cognitive factors further complicate the educational and employment trajectory of many survivors. Particularly limiting is the presence of neurological, sensory, musculoskeletal impairments, and chronic pain associated with survivorship of cancer and associated treatments. Those with such physical limitations (reported as upwards of 20 %) are more likely to require special education services, less likely to graduate high school and be employed, and less likely to have a household income greater than $20,000 [96]. The impact of poor health status (such as pain and neurosensory difficulties) was most limiting in the amount and type of work attainable in this population both in the years immediately following diagnosis and over their working lifetime [101]. These findings remain even after controlling for demographic (e.g., gender), emotional (e.g., depression), and neurocognitive factors [95]. Please see Chapters 24 and 29 for a more in-depth analysis of these issues.
16.4 Neurocognitive Interventions/Cognitive Rehabilitation
16.4.1 Cognitive Rehabilitation
Some attempts have been made to develop non-pharmacologic intervention approaches to addressing the various neurocognitive impairments commonly present in survivors. A tripartite model incorporating rehabilitation, clinical psychology, and special education paradigms has been proposed and is currently being evaluated by Butler and colleagues [102], and is discussed in this manuscript in more detail. Briefly, the model includes individual training in the areas of attention and metacognition. Cognitive-behavioral therapy (CBT) is also provided. Early results indicate some immediate improvement on quantitative neuropsychological measures, although no improvement in academic achievement was shown. This highlights the challenge of developing interventions with ecological validity.
Another emerging therapeutic program that has recently been piloted with pediatric cancer survivors is the Cogmed® program [103]. It is a web-based computer training program specifically targeting working memory impairments. Research to date in children with attention and working memory impairments from various etiologies (e.g., AD/HD, etc.) has revealed improvements in working memory, with sustained benefits to as long as 6-months post-training [104, 105]. Please see Chap. 26 by Dr. Askins and colleagues for a more detailed description of the available cognitive remediation programs.
16.4.2 Pharmacotherapy
A number of trials have evaluated the efficacy of stimulant medications in brain tumor survivors given the similarities in symptomatology to children with developmental attention disorders. There have been mixed results [106–108], although there is some suggestion that methylphenidate in particular may be beneficial in addressing deficits in focus and sustained attention [109–111]. In Conklin’s study, medication response was predicted by higher intellectual ability, male gender, and older age at treatment. There is little to no research to date on the effects of stimulant medication in adult survivors of pediatric brain tumors [111]. The use of pharmacotherapy is discussed in Chap. 27 by Dr. Anna Muriel in more detail.
16.5 Conclusions
Significant gains have been made in the diagnosis and treatment of brain tumors in children. With increasing survival rates, the long-term functional toll of necessary treatments has become an important focus of survival. Appreciation of the individual and treatment-related risk factors has emerged over time, although the majority of research has focused on macro systems of functionality, such as intelligence. Only recently has an appreciation for higher-order neurocognitive factors emerged, and exploring the multifaceted neurocognitive factors involved in diminished functioning in pediatric brain tumor survivors will contribute to improved identification of risk factors as well as more focused interventions. In contrast, no research to date has evaluated neurocognitive protective factors or mechanisms that lead to relatively typical development and functioning.
It has been demonstrated that the failure of a patient’s ability to advance and attain developmentally appropriate goals begins soon after diagnosis of a brain tumor [112], highlighting the importance of early and consistent neurocognitive assessment and intervention. An appreciation of the need for understanding the longer-term outcomes in survivors of pediatric cancers is emerging, but still in its infancy. As many more children are surviving brain tumors and reaching adulthood, they will present with unique challenges, needs, and supports related to these life transitions. They are at elevated risk for complicated medical presentations such as secondary cancers, endocrine deficits, obesity, neurological impairments, neurocognitive deficits, increased fatigue, and poor sleep hygiene [108]. Further, engagement in health maintaining behaviors such as adequate physical activity, dental care and health screenings are reduced in this population [108]. In addition, there is also the significant risk for poor educational and employment attainment, which can reduce their access to appropriate health care resources (i.e., public/government versus private/employer-based insurance). Social-emotional challenges may further reduce the survivor’s ability to adequately advocate for him or herself, limiting independence, and this topic is addressed more fully in other chapters in this text. Many survivors have undergone neuropsychological assessments over time, however, children from smaller, rural areas, and those from disadvantaged backgrounds may not have had access to these services. This places them at greater risk based on limited awareness of long-term sequelae of pediatric brain tumor and limited connections to community resources. Understanding the needs of our pediatric survivors and developing supports and interventions to increase their independence and self-advocacy will be vital in producing more productive, mentally and physically healthy adults.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree