Neuropsychiatric Symptoms and Postacute Sequelae of SARS-CoV-2—The Long Haulers
The previous chapter focused on the pathology and symptomology of COVID-19 but purposely neglected to mention many of the neuropsychiatric symptoms associated with the disease. This chapter will examine these symptoms, as well as how neuroinflammation, chronic systemic inflammation, and psychosocial factors may all converge and define the constellation of neuropsychiatric symptoms frequently reported by patients ranging from those with mild acute cases to those who have experienced persistent problems believed to be related to their initial bout with the disease. This chapter will also include a description of multisystem inflammation syndrome in children (MIS-C) and a discussion of some of the theories that have been proposed to explain the mechanisms of long COVID (or postacute sequelae of SARS-CoV-2 [PASC]), a poorly understood syndrome with a diverse symptomology that may actually be an umbrella term for multiple conditions with heterogeneous etiologies.
COVID-19 and the Central Nervous System
Initial reports about COVID-19 focused primarily on the disease’s severe respiratory symptoms. This should come as no surprise since the reports out of Wuhan in December 2019 characterized COVID-19 as an atypical pneumonia, and some of the first media stories to be associated with the outbreak included an 11-second video clip of a computed tomography (CT) scan showing ground-glass opacities in the lungs of a patient and an image of a report showing a false positive for SARS both taken by Dr Ai Fen, Head of Emergency Medicine at Wuhan Central Hospital.1 Even today, most people tend to associate COVID-19 with respiratory complications, particularly acute respiratory distress syndrome (ARDS).
As research into the pathogenesis of COVID-19 has progressed, evidence has emerged to suggest that it is in fact a multisystem disease that can cause systematic inflammation, blood clotting, organ failure, and sepsis via multiple routes.2 Apart from these system-wide effects, there are also numerous neurological effects of SARS-CoV-2 infection, though the etiology and the route of infection remain unclear. Moreover, it has yet to be elucidated if SARS-CoV-2 directly invades the central nervous system (CNS) or if neurological complications are due to secondary or systemic effects like hypoxemia, immune dysfunction, or coagulopathy.2
Direct evidence of CNS infection was well documented during the SARS-CoV and MERS-CoV outbreaks, indicating that coronaviruses are capable of infecting the CNS; moreover, viral RNA was detected in brain tissue during autopsies of individuals who had tested positive for both viruses.3 SARS-CoV-2 also appears capable of infecting neural tissue and has been detected in the cortical neurons during autopsy,4 as well as in cerebrospinal fluid.5 The route the virus takes to infect the brain remains a mystery, but SARS-CoV-2 may infect the CNS by traveling along nerves from other parts of the body. It is also possible that it may cross the blood-brain barrier after the membrane has been weakened due to the frenzied and systemic inflammatory response, the so-called “cytokine storm” described before (see Chapter 3: Pathology—What We Knew and What We Know).
While direct infection of neural tissue appears possible, it has only infrequently been observed in real-world scenarios. Autopsies of 41 consecutive patients with SARS-CoV-2 infections found low to very low levels of viral RNA in the majority of brains, suggesting that neural infection is not core pathology of COVID-19.6 This would suggest that neuropsychiatric symptoms arise due to a combination of abnormal inflammatory response, hypoxia, and ischemia. Conversely, a study that has yet to be peer reviewed examined the brain scans of 394 COVID-19
patients before and after becoming ill and found deleterious effects to the olfactory and gustatory cortical systems that especially impacted gray matter thickness and volume in the left parahippocampal gyrus, the left superior (dorsal) insula, and the left lateral orbitofrontal cortex. The disease’s impact was also observed in the left cingulate cortex, the left supramarginal gyrus, and the right temporal pole. The 15 patients from the group who were hospitalized due to COVID-19 also showed damage to the right hippocampus and amygdala.7
patients before and after becoming ill and found deleterious effects to the olfactory and gustatory cortical systems that especially impacted gray matter thickness and volume in the left parahippocampal gyrus, the left superior (dorsal) insula, and the left lateral orbitofrontal cortex. The disease’s impact was also observed in the left cingulate cortex, the left supramarginal gyrus, and the right temporal pole. The 15 patients from the group who were hospitalized due to COVID-19 also showed damage to the right hippocampus and amygdala.7
One of the most commonly described neurological symptoms is encephalopathy, which is more common in severe cases of COVID-19.8 Encephalopathy is associated with increased morbidity and mortality, independent of the severity of any COVID-19-related respiratory disease.9 One case series reported encephalopathy to be present in approximately two-thirds of patients with COVID-19-related ARDS,10 while another case series found that 31.8% of hospitalized patients had comorbid encephalopathy.9 Most importantly, a study of 817 older patients in a hospital setting who were diagnosed with COVID-19 infection reported that 226 (28%) had delirium at presentation and 37% of those patients did not present with symptoms typically characterized with COVID-19 (fever, cough, dyspnea, etc). Within the subgroup, 16% presented with delirium as the primary symptom.11 Risk factors for encephalopathy include male sex, old age, cancer, history of neurologic disorder, cerebrovascular disease, heart failure, kidney disease, diabetes, dyslipidemia, hypertension, and smoking.9
Instances of ischemic stroke and hemorrhagic stroke have been reported and may be influenced by a myriad of factors, including coagulopathy, systemic inflammation, and preexisting risk factors typically associated with strokes (hypertension, dyslipidemia, obesity, smoking, etc).12 Despite relatively infrequent occurrence, the phenomenon was given ample attention in the press, especially because reports of COVID-19-related stroke emerged at the height of the first wave of the pandemic in late April 2020 and because those who were affected were often young, otherwise healthy, and ostensibly were experiencing mild cases of COVID-19.13 Despite the small panic that this set off that spring, further studies have found that frequency of stroke is correlated with severity of illness and that patients with mild illness have a <1% risk of having a stroke, while the risk for patients admitted to intensive care units may be as high as 6%.14
Seizures have been reported far less frequently than stroke or encephalopathy, but they still do occur. Moreover, subclinical or electrographic seizures appear to be quite common, especially in critically ill patients.15 Meanwhile, meningoencephalitis and acute encephalitis have been reported in connection with COVID-19, and the former appears to be
rarer than the latter.2 In both instances, patients may present with headache and altered consciousness, especially confusion, with or without respiratory symptoms.16, 17
rarer than the latter.2 In both instances, patients may present with headache and altered consciousness, especially confusion, with or without respiratory symptoms.16, 17
Microglia and Neuroinflammation
CNS is not just composed of neurons; glial cells play a supportive role by helping clear debris, providing structural and synaptic support for neurons, insulating neurons from one another, maintaining the integrity of the blood-brain barrier, and regulating blood flow. Specialized cells known as microglia serve as the resident innate immune system cells of the CNS and are responsible for responding to pathogens through an inflammatory response known as microgliosis.18
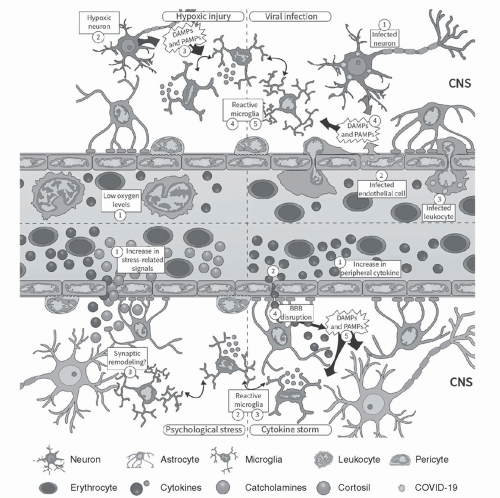
Should a pathogen manage to invade the CNS by breaking through the blood-brain barrier or the blood-cerebrospinal fluid barrier, hitching a ride along an infected peripheral nerve, or another means, microgliosis may also be triggered. In ideal scenarios, microglia respond to these insults in a well-orchestrated process that eliminates injury to the neural tissue with as little damage as possible by releasing a balance of proinflammatory and anti-inflammatory cytokines. However, in other instances, microgliosis may occur without the presence of a pathogen when the insult to the neural tissue may be due to hypoxic brain injury or is activated by systemic cytokines that manage to cross the blood-brain barrier. In other words, even if SARS-CoV-2 is incapable of invading the CNS, it can still indirectly cause neuroinflammation (see Figure 4.1).18
As Gonçalves de Andrade and colleagues very astutely summed up in a paper published in February 2021, “In the context of a cytokine storm or after exposure to chronic psychosocial stress, microglia can become altered in their function and then increase the release of inflammatory mediators, generating pathogenic effects associated with neurological and psychiatric conditions.”18
Neurological Symptoms
As described in Chapter 3: Pathology—What We Knew and What We Know, it is extremely common for patients with COVID-19 to present with nonspecific features of systemic illness that can also manifest as neurological symptoms. A meta-analysis of 215 studies published between January 2020 and July 2020 involving 105,638 patients found that the most common neurological or neuropsychiatric symptoms included anosmia, weakness, fatigue, dysgeusia, myalgia, sleep disorder, depression, and headache (for a full listing, see Ref. 19). One of the study’s limitations is that it overrepresented patients with severe COVID-19, as most of the participants from the 215 studies were recruited from a hospital setting. Consequently, some of the prevalence rates of
individual symptoms may be skewed, but the study still provides an accurate look at the diversity of neurological and psychiatric symptoms associated with COVID-19.
individual symptoms may be skewed, but the study still provides an accurate look at the diversity of neurological and psychiatric symptoms associated with COVID-19.
As noted above, encephalopathy appears to be somewhat common in older patients who may present with delirium and agitation but may also experience tiredness and lapses in memory.8 Similarly, COVID-19-related ischemic strokes or transient ischemic attacks may result in neuronal damage, and symptoms will depend upon the brain region affected by the ischemic event.
Anosmia
Many of the neurological and neuropsychiatric symptoms19 are common during the prodrome of numerous diseases and are not unique to COVID-19. However, the anosmia described by COVID-19 patients has been the focus of intense discussion and research because patients do not report experiencing concurrent nasal obstructions. As anyone who has suffered through a run-of-the-mill rhinovirus infection knows, one’s sense of smell and taste are disrupted because of congestion and blockage. Of note, most COVID-19 patients report no such blockages but still experience significant olfactory dysfunction.
Initially, researchers wondered if this would mean that the virus was capable of infecting the olfactory bulb. More than just a part of the brain that processes sensory information pertaining to scent, the olfactory bulb is one of the physiological antechambers leading to the CNS (in this metaphor, the figurative door would be the olfactory neuroepithelium and the walkway would be the nasal cavity). Consequently, an infection of the olfactory bulb would allow the virus to access the CNS and could potentially lead to infection of the neural tissue.
Criticism of the theory of CNS infection via the olfactory system has focused on the fact that olfactory receptor neurons do not express ACE2 receptors—the primary receptors to which the SARS-CoV-2 virus binds and uses to invade cells. However, ACE2 expression has been observed in the olfactory neuroepithelium (the door from the above metaphor), which could inhibit cell functionality, disrupt olfactory pathways, and lead to partial or total loss of smell.20 Furthermore, SARS-CoV-2 has demonstrated binding affinity with another receptor protein, neuropilin-1 (NRP-1).21 As NRP-1 is highly expressed in olfactory neuroepithelia and the olfactory bulb, it is possible that anosmia results from NRP-1-facilitated infiltration of olfactory neurons.22 Additionally, and far more concerning, is the fact that SARS-CoV-2 may also be able to infect the CNS through the olfactory system. More research is needed to confirm if this is indeed the mechanism behind
COVID-19-related anosmia and if CNS invasion, even if uncommon, can be mediated by infection of olfactory neurons and NRP-1 receptors.
COVID-19-related anosmia and if CNS invasion, even if uncommon, can be mediated by infection of olfactory neurons and NRP-1 receptors.
Guillain-Barré Syndrome
Guillain-Barré syndrome (GBS) is a rare autoimmune disorder in which the immune system targets the body’s peripheral nervous system. Patients suffering from GBS can experience a diverse set of symptoms depending on the severity of the disease that range from feelings of weakness all the way to paralysis, and in extreme cases, individuals with GBS may require mechanical ventilation. However, in most cases, GBS is a temporary condition though some patients may experience prolonged sensations of weakness after recovery.23
Several clinicians have reported concurrent cases of GBS with COVID-19, suggesting that the hyperactive inflammatory response characterized by COVID-19 may trigger GBS. Preliminary evidence suggests that this complication is more common in elderly men but still quite rare (1 or 2 cases per 100,000 adults) and even rarer in children (0.4-1.4 cases per 100,000).24 Additionally, approximately 100 of the 12.5 million Americans who have received the Johnson & Johnson vaccine have reportedly developed GBS. So far, one fatality has been reported as of September 2021.25 Similarly, a study that examined the risk of relapse of GBS after receiving Comirnaty, the Pfizer/BioNTech vaccine, showed that only 1 individual out of the 702 patients previously diagnosed with GBS required brief medical care for relapse of previous syndrome and quickly recovered.26
The Neuroinflammation-Chronic Disease Nexus and COVID-19
As troubling as the word “neuroinflammation” may be, it is unfortunately a relatively common phenomenon. Furthermore, neuroinflammation has been linked with diseases that arise when the body is in a chronic proinflammatory state due to lifestyle choices that include poor diet, lack of exercise, smoking, and excessive alcohol consumption. These choices often manifest as pathologic obesity, hypertension, dyslipidemia, insulin resistance, diabetes, or thrombophilia and increase individuals’ risk of cardiovascular disease or stroke. Research has also found an association between these chronic inflammatory conditions, poor lifestyle choices, and poor gut microbiome health, and research into the association between the microbiome and COVID-19 has found that there is correlation between poor biome health, increased cytokine levels, and worse outcomes with slower recovery from COVID-19.27, 28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree