and Paul Telfer2
(1)
Department of Haematology, Guy’s and St Thomas’ Hospital, London, UK
(2)
Department of Haematology, Royal London Hospital, London, UK
Introduction
The brain is particularly vulnerable to ischemia and an important aim of management is the prevention of cognitive as well as physical disability due to progressive brain damage. Patients who are at risk of neurological complications are not necessarily those who experience frequent painful crises.
The important acute and chronic neurological complications of SCD are presented in Table 4.1 and the anatomy of the major intra-cerebral arteries indicating common sites of vascular damage in Figs. 7.1 and 7.2.
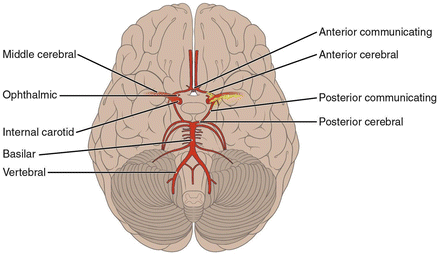
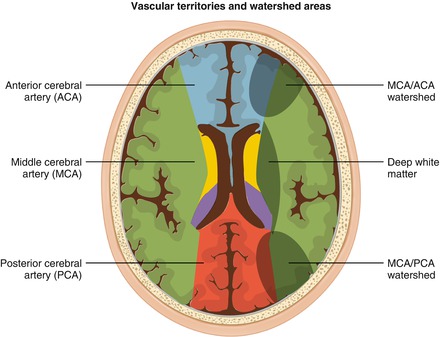

Figure 7.1
The arterial supply of the brain. The common sites of stenotic or occlusive lesions seen in children with SCD are shown (yellow hatched)

Figure 7.2
Transverse section through the cerebral hemispheres at the level of lateral ventricles showing the vascular territories of major cerebral arteries (left side) and ‘watershed’ areas at the borders of these vascular territories which are susceptible to ischemic damage (right side)
Ischemic Lesions
Definitions
For clinical diagnosis, we recommend using definitions developed for use in clinical trials. These criteria require confirmation by a specialist neurologist and neuroradiologist.
Acute Ischemic Stroke (AIS)
History of acute neurological deficit consistent with an arterial territory lesion, and with an acute ischemic lesion in the corresponding vascular territory on magnetic resonance imaging (MRI). Very occasionally, an ischemic lesion is not visible on MRI. This would still be diagnosed as AIS if the neurological deficit was persisting.
Transient Ischemic Attack (TIA)
History of acute neurological deficit lasting less than 24 hours consistent with a lesion in an arterial territory, without a residual neurological deficit, and with no evidence of an acute ischemic lesion on MRI.
Silent Cerebral Infarction (SCI)
Ischemic lesion on MRI (at least 3mm in diameter, seen in two planes) with no clinically detectable neurological deficit.
Etiology and Pathophysiology
Cerebral Ischemia
This is a consequence of failure to maintain adequate brain perfusion. It is assumed that compensatory blood flow around the Circle of Willis is inadequate in the presence of acute anemia, chronic vessel wall damage, and acute vaso-occlusion due to sickled red cells and/or thrombus formation. Ischemic damage is most common in the distribution of the middle cerebral artery (MCA), the watershed region of restricted perfusion between the MCA and the anterior cerebral artery (ACA) or between the MCA and posterior cerebral artery (PCA) (Figs. 7.1 and 7.2).
Cerebral Artery Vasculopathy
Acute ischemic stroke is often (but not always) associated with an arterial vasculopathy characterized by stenotic or occlusive lesions in the large vessels, most commonly the internal carotid artery (ICA) proximal MCA and ACA (Figs. 7.1 and 7.2). The posterior cerebral artery (PCA) and basilar arteries are less commonly and less severely affected. Histological examination of the vessel wall shows intramural thrombus which is often organized and re-canalized. The vessel wall is damaged with endothelial disruption, duplication of the internal elastic lamina and scarring of the tunica media (Fig. 7.3). These vascular lesions often arise at areas of turbulence. It is here that shearing forces caused by abnormal sickled red cells flowing over the endothelium are most likely to cause physical damage and provoke inflammation.
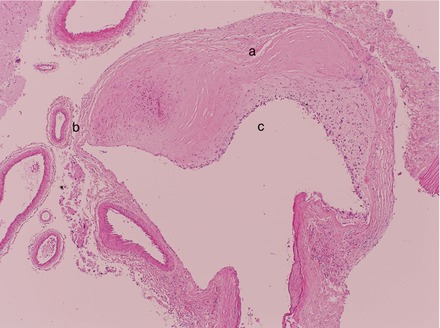

Figure 7.3
Post mortem histological section through parts of a major intracerebral artery showing areas of thickening (a) and thinning (b) of the vessel wall. The vessel lumen is also narrowed (c)
Vasculopathy can be progressive, leading to large vessel occlusion and new vessel formation. Moyamoya is a term used to describe a particular appearance of abnormal vasculature with vessel dilatation and new vessel formation. Moyamoya vessel formation implies a worse prognosis for stroke, silent ischemia and cerebral hemorrhage. Moyamoya changes are visible on cerebral MRI (Fig. 7.4) and Magnetic Resonance Angiography (MRA) although direct angiography is required for confirmation of findings and for planning surgical or invasive radiological intervention (Fig. 7.5a, b).
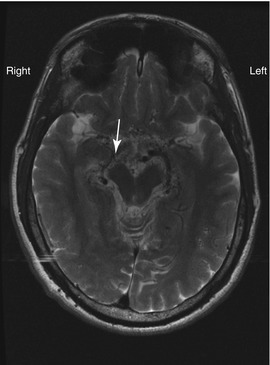
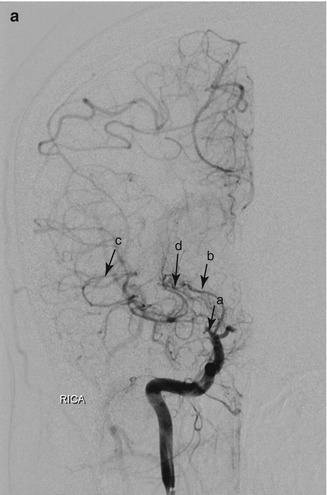
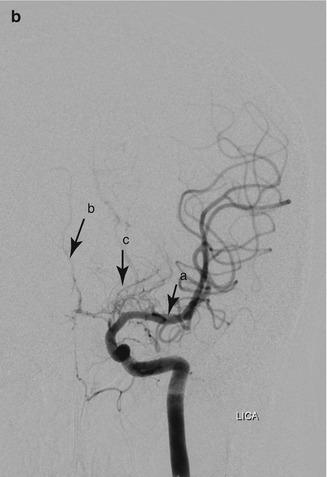

Figure 7.4
T2 weighted cerebral MRI of adult male. The MCA is occluded and not visible on the right side. Moyamoya vessels in section are seen as numerous black dots (flow voids) in the region of the midbrain (arrow)


Figure 7.5
(a) Moyamoya in a 20 year old woman with HbSS. Digital subtraction angiogram with contrast injection into the right internal carotid artery. The right middle cerebral artery is occluded near its origin (a), distal to the proximal lenticulostriate artery (b), which is filling the distal middle cerebral artery and its tributaries (c) through the distal lenticulostriate arteries (d). (b) Digital subtraction angiogram with contrast injection into the left internal carotid artery (same patient as a). In contrast to the right side, this shows normal MCA (a) but near-absent filling of the left anterior cerebral artery. Very faint filling of the ACA is seen (b) through moyamoya collaterals (c). RICA right internal carotid artery, LICA left internal carotid artery
Vasculopathy can also affect the carotid and vertebral arteries in the neck (Fig. 7.6). Lesions in the cervical portion of the ICA have been associated with ischemic stroke involving the territories of the MCA and ACA, as well as progressive silent ischemia in the anterior watershed distribution.
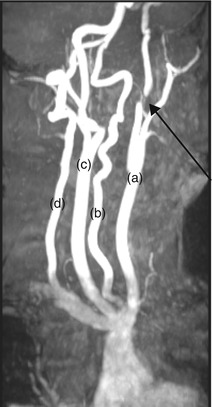

Figure 7.6
MRA of major cervical arteries. Oblique view showing occlusion of left ICA just beyond the bifurcation of the common carotid artery (arrow). The left common carotid (a), left vertebral artery (b), right common carotid (c), and right vertebral arteries (d) can be seen. A common incidental finding is abnormal tortuosity of the vertebral arteries
Radiological Investigation and Findings
Cerebral magnetic resonance imaging (MRI) is the investigation of choice for suspected ischemic lesions. These appear as hyper-intense (white) areas on T2-weighted sequences. FLAIR (Fluid Attenuated Inverse Relaxation) increases the conspicuity of ischemic lesions as the fluid signal from CSF in the ventricles and around the brain is nulled out and appears hypo-dense (black). T2 FLAIR images are often used for accurate determination of the position and size of infarcts (Fig. 7.7a) and are essential for diagnosis of silent ischaemia. In the acute setting, diffusion weighted imaging is useful for identifying areas of acute cell swelling in the early stages of ischemia (Fig. 7.7b).
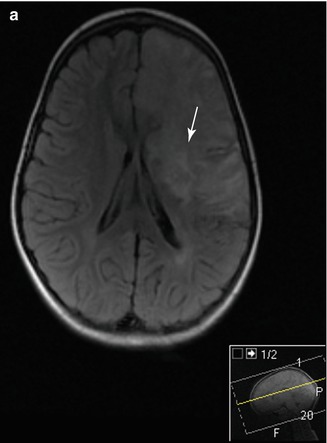
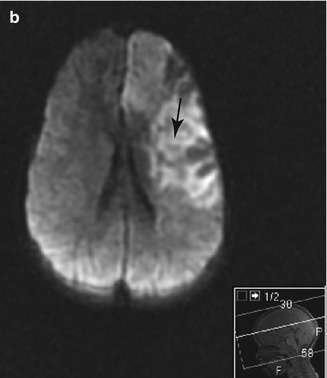


Figure 7.7
Cerebral MRI of a 4 year old child presenting with R hemiparesis resulting from an acute ischaemic stroke with left ICA occlusion. (a) T2 FLAIR image showing a large high intensity lesion in the territory of the ACA and MCA with involvement of the cortex and basal ganglia (arrow). (b) Diffusion weighted image (DWI) showing high signal in the same areas, consistent with acute ischemia (arrow)
Computerized tomography (CT) is commonly used in emergency departments and is the preferred imaging method for acute cerebral haemorrhage. For suspected acute ischemic events, head CT should be followed by magnetic resonance imaging.
Magnetic resonance angiography (MRA) is an important non-invasive method for demonstrating stenotic or occlusive lesions in the large arterial vessels of the head and neck. MRA identifies reduced or turbulent blood flow within the vessel lumen and is therefore an indirect method of demonstrating stenosis or occlusion. More refined MRI techniques can be used to image the vessel wall directly (for instance to demonstrate a dissection or intra-luminal thrombus).
CT Angiography and Direct Four Vessel Contrast Angiography
These techniques are preferred for accurate determination of the location and size of vascular lesions and aneurysms. They are important for defining the vascular anatomy and extent of collateral flow prior to undertaking surgical bypass procedures or interventional neuroradiology.
Acute Ischemic Stroke (AIS)
Clinical Presentation
Patients may present with typical features of stroke: hemiparesis or single limb weakness, hemi-facial droop, hemi-anopia or dysphasia. Sometimes the presentation is less obvious, with transient diminished motor function, loss of consciousness, confusion, or behavioural change. Usually the presentation is purely neurological, but AIS can also be a complication of a severe vaso-occlusive crisis, most commonly a severe acute chest syndrome, or an acute anemic event. In the presence of an antecedent crisis, the risk of recurrent stroke is lower. There have also been reports of events occurring after varicella zoster, parvovirus, and other acute infections.
Assessment and Investigations
Apart from a detailed neurological examination, repeated periodically to determine progression or regression of neurological signs, it is important to assess for evidence of additional sickle complications, as well as for infection, obstructive sleep apnea, abnormalities in blood pressure, and oxygen saturation. Laboratory tests (Table 4.3) are important for excluding an acute anemic event, for compatibility testing and for determining HbS% prior to urgent exchange transfusion. Low steady state hemoglobin, and raised white cell count are risk factors for AIS, but do not guide treatment in the acute situation. Radiological investigations recommended for investigating an acute neurological event are listed in Table 7.1.
Table 7.1
Recommended investigation of acute neurological event
Investigation | Purpose |
|---|---|
CT brain | Emergency diagnosis of AHS if MRI unavailable at presentation. (Ischaemic changes may not be apparent in the first few hours after the event) |
Cerebral MRI: T1- and T2-weighted, FLAIR and DWI. | Confirmation and anatomical localization of acute hemorrhagic and ischemic stroke. Exclusion of other acute neurological disorders including PRES |
Cerebral and cervical MRA. Fat-saturated imaging of cervical ICA. Magnetic resonance venogram. | Demonstration of intracerebral arterial occlusion(s). Demonstration of chronic vasculopathic lesions (e.g. moyamoya). Further investigation of suspected extracranial arterial stenosis/occlusion/dissection or cerebral venous sinus thrombosis if clinically indicated |
Echocardiography | Exclusion of intra-cardiac thrombus and patent foramen ovale |
Incidence
The incidence is highest in HbSS, and much lower in HbSC and HbS β thalassaemia. For acute infarctive stroke, the highest incidence rate is in children in the age range 2 to 9 years (between 0.5 and 1 per 100 patient years follow-up). The rate is also high in adults over the age of 40. Implementation of TCD screening programmes has significantly reduced the incidence of AIS as we shall see later in this chapter.
Management
AIS is a medical emergency. The objectives of treatment are to minimise ischemic damage, improve perfusion of viable tissue and prevent recurrence. Initial management should ensure hemodynamic stabilization, adequate hydration, and normalization of oxygenation saturation. Although there are no trials evaluating transfusion in AIS, exchange transfusion has become standard practice with the objective of optimizing Hb level and reducing HbS percentage urgently. If the Hb is <70 g/l top-up transfusion should precede exchange. The target HbS is <30 %, and Hb level should not be allowed to exceed 110 g/l.
There have been no trials evaluating the use of aspirin, anticoagulation or thrombolysis in acute management of AIS. These interventions are not necessarily beneficial, since the pathophysiology of AIS in SCD is different from thromboembolic stroke in the elderly, where these treatments are well established. We recommend that adults are considered on an individual basis if presenting within the therapeutic window for thrombolysis.
In the UK, adult SCD patients could be managed on a specialized acute stroke unit, and it is advisable to formulate a local protocol with stroke physicians for imaging investigations, acute treatment and rehabilitation. AIS in older patients may be related to cerbrovascular risk factors which apply to the general population. These patients should be discussed with a stroke physician to decide if sickle or non-sickle risk factors are predominant and whether they should be treated initially with thrombolysis or exchange transfusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree