Fig. 1.1
MRI scan of the thoracic spine. At the T6 level, the epidural tumor (outlined) is causing impending compression of the spinal cord (arrow)
As a tumor invades the vertebral bodies , it induces activity of inflammatory mediators within the bone and soft tissue, which causes edema, venous stasis, and finally, ischemia at the level of compression. Once the tumor mass has expanded enough to cause venous congestion, an extensive inflammatory cascade ensues, causing edema of the spinal cord. If treated expediently using corticosteroids, this can be reversed. Corticosteroids are used to treat both the edema and the inflammation and, when used acutely, may ameliorate these processes. If they are left untreated, ischemia and demyelination are likely.
Cortical bone destruction in vertebral bodies does not occur until late in the disease process. The level of bone destruction must reach 30–70 % before it can be seen on plain X-rays. Bone destruction may cause a compression fracture of a vertebral body and retropulsion of bone fragments into the spinal canal, leading to mechanical compression of the spinal cord.
Clinical Manifestations and Findings
The presenting symptom of malignant spinal cord compression in about 90 % of cases is back pain. Although back pain is a common acute problem in the general population, in patients with a history of cancer, it must elicit a high degree of suspicion to ensure an early diagnosis. Pain associated with malignant spinal cord compression is often exacerbated by an axial load or associated with radicular symptoms . Pain that worsens while the patient is recumbent is unusual in those with degenerative disc disease and should raise the concern that the patient has epidural metastasis. Most often, the pain occurs at the area of vertebral compression. It is often described as gnawing or aching pain and is worse during the Valsalva maneuver. Palpation and percussion down the spine frequently help localize metastatic deposits. The pain is either unilateral or bilateral depending on the level of disease. Thoracic involvement frequently results in bilateral symptoms, whereas unilateral pain is seen with cervical or lumbosacral involvement. Complaints of thoracic pain should especially arouse suspicion, as disk herniation and spinal stenosis occur infrequently at this location. Pain while the patient is in the recumbent position worsens owing to lengthening of the spine and distension of the epidural venous plexus. Pain during motion usually is caused by vertebral body collapse and can be associated with spinal instability. Pain may precede neurologic symptoms by several weeks, so early intervention prior to the development of incontinence or inability to walk is one of the most important variables in a successful outcome aside from elimination of the primary tumor.
The second most common symptom of malignant spinal cord compression is weakness , which is present in 35–80 % of patients. Weakness is often associated with corticospinal tract signs such as hyperactive deep tendon reflexes, spasticity, and extensor plantar responses. Weakness is an ominous finding that, if not investigated, may lead to complete loss of spinal function below the level of the lesion.
Leg ataxia may be present before weakness arises and may occur without pain. Using a standardized strength scale (Table 1.1) during the initial evaluation greatly aids in monitoring the clinical course of the patient’s disease. Each muscle group should be tested separately, and the results for both sides of the body should be compared. Rectal sphincter tone should be checked in all patients suspected of having malignant spinal cord compression. Patients who are immunosuppressed or at risk for bleeding can be safely tested by placing a gloved finger adjacent to but not in the anal canal while the patient attempts to tighten the anal sphincter. A simple observation of the umbilicus can detect a spinal cord injury between the T10 and T12 levels. Known as the Beevor sign , this is done by having the recumbent patient flex his or her head against resistance. The umbilicus moves cephalad if the involvement is below the T10 level.
Table 1.1
Standardized muscle strength scale
Rating | Strength |
|---|---|
0/0 | No contraction |
1/5 | Muscle flicker, but no movement |
2/5 | Movement possible with gravity eliminated |
3/5 | Movement possible against gravity but not against resistance by the examiner |
4/5 | Movement possible against some resistance by the examiner |
5/5 | Normal strength |
The Babinski sign is a sensitive, specific sign of corticospinal tract dysfunction, but interpretation of this valuable sign requires experience. Although most clinicians observe the great toe’s movement during noxious stimulation along the lateral aspect of the bottom of the foot, the movement of the four smaller toes is a more reliable indicator. As Babinski observed, “The toes, instead of the flexing, develop an extension movement at the metatarsal joint.”
Diagnosis
Diagnosis of malignant spinal cord compression begins with obtaining a thorough medical history and performing an appropriately focused physical examination coupled with a full central nervous system examination. New onset of back pain or neurologic symptoms , such as symmetric weakness and paresthesia, in a patient with known cancer should prompt further work-up for malignant spinal cord compression.
Magnetic resonance imaging (MRI) has a sensitivity rate of 93 %, specificity rate of 97 %, and overall accuracy rate of 95 % in revealing spinal cord compression. In the absence of contraindications or intolerance, MRI is usually sufficient in investigation of malignant spinal cord compression. Because one third of patients have multiple sites of compression, many researchers recommend imaging the entire spinal cord or, at minimum, the thoracic and lumbar spine. The study takes about 45 min and requires the patient to fit into an MRI scanner, lie flat, and be absolutely still.
Computed tomography (CT) myelography is a helpful technique for patients who cannot undergo MRI (e.g., those with pacemakers or extreme claustrophobia). It facilitates assessment of osseous integrity as well as the thecal sac contents and has the added benefit of allowing for cerebrospinal fluid (CSF) sampling at the same time. Disadvantages of CT myelography include its overall greater cost than that of other available imaging tests, its invasive nature and inherent risk of contrast reaction, and postprocedure spinal tap-related headaches.
Plain X-rays , although expedient and inexpensive, are not useful in the initial evaluation of suspected malignant spinal cord compression. They are not positive for compression until nearly 70 % of the bone is destroyed, which usually occurs at a late stage in the evolution of symptoms.
Bone scanning and positron emission tomography using [18F]fluoro-2-deoxy-2-d-glucose are not useful in detecting cord compression, although both do demonstrate bony metastases.
Treatment
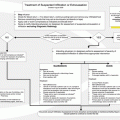
Because malignant spinal cord compression is associated with advanced-stage cancer, all treatments of it are palliative in nature and consist of pharmacotherapy, surgery, radiotherapy (RT), or a combination of them. The goals of therapy for malignant spinal cord compression should include (1) preservation of function and mobility, (2) pain relief, (3) local tumor control, and (4) spine stability.
Corticosteroid-based therapy should be administered in cases with a suspicion of cord compression and in which myelopathy is observed. Pain, which is difficult to control in the absence of neurologic symptoms, also may be an indication for steroid use. Steroids interrupt the inflammatory cascade, leading to a reduction in vasogenic edema. Pain and neurologic symptoms often improve afterward, which can be a prognostic indicator as to how well the patient’s disease may respond to therapy.
Studies of acute spinal cord injury have suggested marked neurologic improvement with the use of steroids within 8 h after injury. In a randomized controlled trial, researchers compared high-dose (100-mg loading dose, then 96 mg daily) and moderate-dose (10-mg loading dose, then 16 mg daily) dexamethasone. They found no differences in efficacy; thus, most physicians give the lower dose. Tapering of steroids is begun as soon as feasible to avoid steroid-associated complications such as hyperglycemia, insomnia, and gastrointestinal irritability. The last of these side effects is common and should be treated with antacids. A lesser known but more serious complication is lower intestinal perforation, which can be minimized by preventing the patient from becoming constipated and using the lowest possible dose of steroids. In patients presenting with undiagnosed spinal masses and no history of cancer, especially young patients, steroid use should be avoided until diagnosis. Steroids have an oncolytic effect on some tumors, particularly lymphomas and thymomas, which may delay diagnosis.
Pain may be relieved by the administration of steroids, but often, additional analgesics are required. This can be a major focus of treatment. Using the World Health Organization’s analgesic ladder, a physician can choose the most appropriate medication on the basis of the severity of the pain.
In the absence of bony instability, RT has historically been the treatment of choice for malignant spinal cord compression, preferably started within 24 h of diagnosis. This requires a prompt consultation with a radiation oncologist . Radiation is usually fractionated over a few days to weeks to minimize its harmful effects on normal tissue. Pain is often improved with RT, and further tumor growth and neurologic damage are prevented. Neurologic outcome, with the goal of ambulation following RT, depends on the patient’s ambulatory status at the time of diagnosis, timing of treatment (i.e., started within 12 h after presentation), presence of a single metastatic tumor, and severity of cord compression. Patients with radiosensitive tumors , such as lymphomas, myelomas, and breast and prostate cancers, are more likely than those with less radiosensitive tumors to regain neurologic function after RT. About 90 % of ambulatory patients retain ambulation after RT alone, but less than 30 % of patients who have lost the ability to walk by the time RT is initiated regain ambulation.
Anterior vertebral body resection with stabilization may offer the best chance for a good outcome, but the procedure is a major undertaking and requires (1) a good performance status, (2) uninvolved adjacent vertebral bodies for stabilization of the spinal canal, and (3) a skilled neurosurgical team.
Emerging treatment options such as stereotactic radiosurgery and vertebroplasty may provide some symptom relief for patients who are not surgical candidates.
Summary
Malignant spinal cord compression is a neurologic emergency frequently seen in cancer patients. Even patients with advanced disease and limited life expectancy can benefit from prompt therapy when it is appropriate for their circumstances. Prompt recognition and treatment of malignant spinal cord compression by a multidisciplinary team offer the best outcomes for these patients.
Seizures in Cancer Patients
Patients with cancer have a higher incidence of seizures than that in the general population (Fidler et al. 2002). Prolonged convulsive seizures in cancer patients can lead to brain injury, rhabdomyolysis, renal failure, and death. The discussion below focuses on definitions, evaluation, etiologies, and management of prolonged seizures in adult and pediatric patients with cancer presenting to the emergency center (EC).
Definitions
Early reports on SE defined it as “whenever a seizure persists for a sufficient length of time or is repeated frequently enough that recovery between attacks does not occur.” Many authors have defined this length of time as 30 min because experimental studies demonstrated that irreversible neuronal damage occurs after this period (Sperduto et al. 2008). However, most physicians would agree that treatment of SE should begin before 30 min elapse. Lowenstein and Alldredge (1998) proposed a revised definition of SE as “either continuous seizures lasting at least five minutes or two or more discrete seizures between which there is incomplete recovery of consciousness.” This is the definition that is generally accepted today (DeAngelis and Posner 2009). This definition aims for rapid initiation of antiepileptic administration because controlling convulsive SE earlier rather than later is beneficial. Time is of the essence.
Also, a consensus on the definition of refractory SE is lacking. One suggested definition is failure of 2 or 3 anticonvulsants combined with a minimal duration of the condition of 1 or 2 h or regardless of the time elapsed since onset (Sperduto et al. 2008). Another definition is seizures lasting more than 2 h or recurring at a rate of 2 or more episodes per hour without recovery to baseline between seizures despite treatment with conventional antiepileptics (Groves 2010).
The definition of nonconvulsive SE (NCSE) is based on changes in behavior and/or mental processes from baseline that are associated with continuous epileptiform discharges on electroencephalograms (EEGs) (Groves 2010). Unfortunately, agreement regarding the duration that these alterations must be present is lacking, but most physicians would consider any abnormal epileptiform discharges on an EEG to warrant treatment.
Evaluation of a Cancer Patient with Seizures
When evaluating cancer patients with seizures, understanding the different etiologies of seizures is important. Most seizures in cancer patients are attributed to brain metastasis, but they can also be secondary to other abnormalities , such as intracranial hemorrhage and radiation necrosis. Cancers that commonly metastasize to the brain include breast and lung cancers and melanoma. Patients with primary brain tumors are also at risk for seizures. Other causes of seizures include metabolic abnormalities, infection, hypoxia, and medications that lower the seizure threshold.
Reversible posterior leukoencephalopathy syndrome can occur in cancer patients for a variety of reasons. It is associated with severe hypertension, altered mental status, and posterior cerebral T2 signals on MRI scans. Patients may present with headache, confusion, seizures, and visual impairment. Lowering the patient’s blood pressure and discontinuing use of the offending agent often will prevent seizure reoccurrence. The agents most commonly associated with this syndrome include cyclosporine, tacrolimus, sirolimus, rituximab, cytarabine, etoposide, cisplatin, oxaliplatin, gemcitabine, methotrexate, intrathecal chemotherapeutics, interferon-α, antiretroviral therapeutics, and high-dose methylprednisolone (Fidler et al. 2002).
Diagnostic Testing
Work-up for seizures should begin with a complete neurologic examination and history from a witness or family member of the patient. Laboratory values, including electrolyte, glucose, calcium, magnesium, phosphorous, and creatine kinase levels; complete blood count; and hepatic and renal function, should be obtained immediately. If indicated, arterial blood gas and antiepileptic medication levels may be measured, and echocardiograms, EEGs, and drug screens may be performed.
CT and MRI are indicated for patients with cancer who have seizures. MRI is preferred; however, CT is often performed because of its ability to quickly rule out intracranial hemorrhage. If possible, a contrast agent should be administered intravenously to help evaluate the patient for metastasis and abscesses. Lumbar punctures are indicated when an infection is suspected in the presence of fever or an elevated white blood cell count, which may be difficult to assess in cancer patients.
Management
Initial management of seizures should begin with assessing the patient’s airway, breathing, and circulation. Intubation may be required if the patient has a compromised airway or severe hypoxemia. If the patient is hypoglycemic, he or she should receive 50 mL of dextrose 50 % in water. SE should be treated immediately with intravenous (IV) benzodiazepines. Studies have demonstrated lorazepam to be superior to diazepam, and pharmacokinetic studies have demonstrated that the anticonvulsant effect of lorazepam lasts much longer than that of diazepam (Groves 2010).
In addition, administration of a long-acting anticonvulsant should be started simultaneously. Phenytoin (PHT) or valproic acid is usually indicated; these two agents have the most evidence supporting their use. Unfortunately, these older generation medications may interact with chemotherapeutics and have unwanted cardiovascular side effects. This should not preclude their use given the patient’s acuity and the need for controlling this unstable situation. Other agents, such as levetiracetam (LEV) and lacosamide, are frequently used, but data supporting their efficacy in patients with SE is lacking. In a recent retrospective study of 23 patients with primary or metastatic brain tumors who had SE, all of the patients were given IV PHT and LEV and oral pregabalin. SE was resolved in 70 % of the patients, with only one of the responders needing intubation. Although this study had many limitations, it provides insight into a regimen that may be safe and effective for seizures in patients with brain tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree