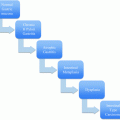
Grade
Mitotic count (10HPF)a
Ki-67 index (%)
G1
<2
≤2
G2
2–20
3–20
G3
>20
>20
Imaging
Radiographic imaging is crucial in the evaluation of NETs and in determining optimal surgical management. Contrast-enhanced CT and MRI have the most clinical utility in assessing the primary tumor and the extent of malignant disease, given the excellent sensitivity and specificity of these tests.
Functional imaging, most notably Somatostatin Receptor Scintigraphy (SRS , Octreotide scan) is also utilized, but has lower accuracy compared to multiphase CT and dynamic MRI. In SRS, a radiolabeled somatostatin analog (classically Indium-111-DTPA octreotide) is injected intravenously and localizes in tissues dense with somatostatin receptors (SSTRs), specifically SSTR2 [9]. 68Ga-labeled somatostatin analogs have also become increasingly beneficial in clinical practice. The half-life for these analogs is only 1 h, so imaging studies can be completed in 2–3 h, compared to between 24 and 48 h for SRS [12]. Additionally, less than half of the dose compared to that required for Indium-111-DTPA octreotide is needed, reducing patient risk [13]. Combination of single photon emission CT (SPECT) and SRS to give fusion images can enable clinicians to correlate SSTR activity with anatomical position [10].
SRS can also be useful in treatment planning and in therapy. Gastrointestinal NETs often have a high affinity for somatostatin and its analogs because of high expression of SSTRs. This high expression can be confirmed with SRS, which can be helpful in deciding on somatostatin analog therapy. Somatostatin analogs are used for therapy when surgery is not possible or distant metastases render it ineffective. Administration of this therapy to patients has become less cumbersome as slow depot injections have been developed for octreotide (octreotide long-acting repeatable or LAR) and lanreotide (lanreotide slow release or SR) [15]. The tumor static effects of somatostatin analogs entail a variety of mechanisms, including the inhibitory action of activated SSTR2 and SSTR5 on the mitotic cycle in neoplastic lesions [16]. Additionally, somatostatin analogs seem to have an anti-angiogenesis effect on neoplastic lesions, inhibiting tumor growth via inhibition of vascular endothelial growth factor 1 [17]. Peptide receptor radionuclide therapy (PRRT) is the use of radioactive isotopes bound to somatostatin analogs like octreotide or lanreotide to irradiate neuroendocrine neoplastic tissue [14].
Positron emission tomography (PET) is increasingly being utilized in NET diagnosis. Fluorine-18 (18F)-fluorodeoxyglucose (F-FDG) PET may be more sensitive than SRS for aggressive tumors with proliferative index >15 [10]. F-FDG PET may also have prognostic utility with a maximum standardized uptake value (SUVmax) > 3 predictive of progression-free survival [11].
Foregut
Foregut carcinoids include NETs of the stomach, duodenum, and pancreas (PNETs). In general, foregut carcinoids appear trabecular histologically and argyrophil silver staining is employed for microscopic differentiation. Each of the foregut NETs has individual characteristics, outcomes, and surgical options worth considering.
Stomach
Unlike most other GI NETs, gastric carcinoids often occur in association with another disease process, rather than sporadically. These tumors are traditionally divided into three types, two of which are associated with concurrent medical conditions. including chronic atrophic gastritis, pernicious anemia (type 1), MEN1, and Zollinger-Ellison Syndrome (ZES) (type 2). Chronic atrophic gastritis is a condition where gastric parietal cells do not secrete adequate amounts of hydrochloric acid (HCl) or intrinsic factor, leading to decreased absorption of vitamin B12 in the terminal ileum and a resulting pernicious anemia [18]. The achlohydria stimulates gastrin release from G cells in the antrum, initiating a trophic effect on gastric enterochromaffin-like (ECL) cells, the most common gastric neuroendocrine cell type, which become hyperplastic and potentially neoplastic gastric carcinoids [19]. Type 2 gastric carcinoids are uncommon, often associated with MEN1 or ZES, conditions in which G cells in the duodenum or pancreas become hyperplastic and secrete high levels of gastrin (gastrinoma). The resulting hypergastrinemia leads to ECL cell hyperplasia, which can progress to a potentially neoplastic lesion [20]. Type 3 gastric carcinoids , on the contrary, arise sporadically and are not associated with elevated gastrin levels. Type 3 tumors are larger, more invasive, and have a greater metastatic potential (>50 %) when compared to types 1 and 2 (Table 2).
Table 2
Recommended surgical management of gastric carcinoid tumors
Comparison of the three types of gastric carcinoid tumors | |||
|---|---|---|---|
Type I | Type II | Type III | |
Frequency | 70–80 % | 5–10 % | 15–20 % |
Associated conditions | Pernicious anemia | ZES, MEN1 | None (most are sporadic) |
Gastrin level | Increase | Large increase | No change |
Tumor characteristics | Multicentric, <2 cm | Multicentric, <2 cm | Solitary, >2 cm |
Metastatic potential | <5 % | 7–12 % | >50 % |
Treatment | Endoscopic resection and surveillancea | Endoscopic resection and surveillancea, gastrinoma resection | Formal surgical resection |
Type 1 gastric carcinoids can traditionally be treated endoscopically. In some cases, mitigation of hypergastrinemia is achieved by the removal of gastric G cells via antrectomy, but 14 % of patients may still see a progression in tumor growth [21]. In general, because of the low metastatic potential of type 1 (and 2) gastric carcinoids, prognosis is good. For type 2 gastric carcinoids, management is very similar to type 1 unless the patient has MEN1 syndrome, in which case surgical removal of the gastrinoma is indicated. Because MEN1 syndrome is a condition of neoplastic endocrine tissue, these gastrinomas are often diffuse and sometimes require radical resection of gastric and duodenal G cells [22]. Type 3 gastric carcinoids are larger and more invasive in nature with more metastatic potential, and therefore require a formal oncologic resection with regional lymphadenectomy for their treatment [20].
Duodenum
Duodenal carcinoids are traditionally limited to mucosal and submucosal tissue and do not include duodenal G cell gastrinomas, which are most commonly found in the duodenum but are classified with pancreatic neuroendocrine tumors. Unlike gastric carcinoids, duodenal NET prognosis and surgical treatment options are related almost exclusively to size. Tumors smaller than 1 cm are traditionally treated via endoscopic resection [23], while tumors measuring between 1 and 2 cm are subject to transduodenal excision [24].
For duodenal carcinoids greater than 2 cm in size, more aggressive surgical treatment is indicated but the precise procedure has not been made clear. At a minimum duodenal segmental resection with lymph node dissection should be performed in order to obtain complete resection of the primary tumor and to provide adequate lymph node harvest for staging (defined as at least 12 lymph nodes). Some have reported that partial gastrectomy maybe is necessary [25], but this should only be performed to obtain negative margins. The extent of surgical resection should be determined by tumor grade, size, and location in that order. An important distinction should be made for tumors originating from the ampulla of Vater, as these lesions can have nodal metastases at a very small size. For mobile, superficial lesions where negative margins are obtainable, ampullectomy can be performed; otherwise, pancreatoduodenectomy is indicated [26].
Pancreas
The inherent endocrine function of the pancreas makes it a popular site for neuroendocrine tumors, called pancreatic NETs (PNETs). Essentially any hormone-secreting cell of the pancreas can become neoplastic, the most common being insulinomas, gastrinomas, glucagonomas, VIPomas, and somatostatinomas. PNETs are referred to as functional when they are associated with a clinical diathesis as a result of their hormonal hypersecretion, or nonfunctional when no clinical syndrome is apparent. Nonfunctional PNETs still may hypersecrete enteric hormones including chromogranin A, synaptophysin, and pancreatic polypeptide. While the majority of PNETs are sporadic in nature, some conditions are associated with a higher incidence, including ZES, MEN1, von Hippel-Lindau disease (VHL), neurofibromatosis 1 (NF1), and tuberous sclerosis [27].
Insulinoma
Insulinomas are the most common type of functional PNET. Symptoms of insulinoma are related to the hypersecretion of insulin, leading to episodes of hypoglycemia, neuroglycopenia, diaphoresis, tremor, and palpitations. Whipples triad of low blood glucose, symptoms of hypoglycemia, and relief of symptoms with treatment of hypoglycemia are classically suggestive of disease. Diagnosis can be confirmed with a monitored fast (up to 72 h), documenting high insulin levels in the setting of hypoglycemia (a high insulin:glucose ratio) as well as elevated C-peptide levels and negative urine test for sulfonylureas to rule out exogenous insulin administration [28]. Preoperative analysis and localization can be difficult in insulinomas because they are small and lack the high density of somatostatin receptor type 2 (SSTR2), rendering SRS less clinically useful. Instead, pancreatic protocol CT combined with an endoscopic ultrasound (EUS) has up to 90 % sensitivity in localizing insulinomas [29].
Surgical removal is the best treatment option for insulinomas. Most insulinomas are benign (90 %), solitary, and small, making ablation and enucleation therapies ideal. Case studies have shown patients with severe hypoglycemic episodes have uneventful postoperative recoveries and total resolution of their hypoglycemic symptoms [30, 31].
Gastrinoma
Gastrinoma, commonly referred to as Zollinger Ellison Syndrome (ZES), is the second most common sporadic PNET but the most common PNET for patients with MEN1. Gastrinomas are often malignant on presentation, and are typically multiple in MEN. Patients with recurrent, diffuse, or severe peptic ulcer disease should be evaluated for an underlying gastrinoma. Diagnosis is classically made if IV secretin is administered and serum gastrin levels rise to >200 pg/mL above baseline [32].
ZES is also diagnosed with elevated gastrin levels (>100 pg/mL) in the setting of a basal acid output of >15 mEq/h.
Before surgical intervention, acid secretion is often controlled with proton pump inhibitors. For gastrinoma localization, SRS in combination with CT or MRI offers 80 % sensitivity and 100 % specificity. For patients with very small gastrinomas in the pancreas, EUS is an alternative if SRS with CT or MRI is inconclusive. Most gastrinomas (83 %) are located in the gastrinoma triangle, along the first three portions of the duodenum and the head of the pancreas [33]. These tumors are most commonly in the duodenum and are traditionally small. One study documented 64 cases of duodenal gastrinoma, 39 of which (61.9 %) measured <1 cm [34].
Surgical procedure depends on location and size of the gastrinoma. Gastrinomas located in the head of the pancreas can be traditionally enucleated, while those in the body or tail undergo distal pancreatectomy [32]. Duodenotomy or duodenal transillumination with intraoperative endoscopy to explore for tumors is recommended for both pancreatic and duodenal gastrinomas, particularly in MEN, because of small size and this common location. One study compared a group of patients with and without routine duodenotomy and documented that 68 % of patients undergoing duodenotomy had a survival rate of 52 %, compared to patients without a duodenotomy with 26 % survival [35].
Management of gastrinoma in the setting of MEN1 has historically been controversial, given its diffuse nature and proclivity for recurrence. Current recommended management strategy includes resection of disease to prevent development of distant (hepatic) metastases and to prolong expected survival.
Nonfunctional PNET
Overall nonfunctional PNETs are the most common neuroendocrine tumors of the pancreas. Although sporadic or nonfunctioning PNETs account for <2 % of pancreatic tumors, their incidence is increasing over the past decade, seemingly due to increased utilization and quality of axial imaging [36, 37]. Management of the incidentally discovered, asymptomatic PNET less than 2 cm in size is controversial given the unknown and variable malignant potential, with some advocating surveillance and others advocating resection citing malignant behavior even in small tumors. Nonfunctional PNETs demonstrate significant cytological and morphological heterogeneity. They also demonstrate significant prognostic variability making treatment decisions and clinical pathways challenging. Most studies agree that the presence of distant metastasis is the single most important adverse prognostic factor. After that, it has been validated by various authors that in locoregional disease, the strongest predictors for survival are disease stage and tumor grade. In general, morphologic examination is performed to distinguish well-differentiated, low-grade tumors from poorly differentiated, high-grade tumors [38]. A recent international cohort study on PNETs reported a 10-year survival rate of nearly 90 % after resection of G1 tumors compared to less than 25 % for G3 tumors [39].
Proliferation is determined by measuring the number of mitoses per microscopic high power field and Ki-67 index [36, 41, 42]. The latest iteration of the WHO classification includes Ki-67 index reporting for grade [40]. The Ki-67 index is related to the malignant potential and clinical behavior of PNETs and may be an important prognostic indicator, predicting overall and disease-free survival [40, 43–45]. In addition, an adequate lymph node evaluation, defined as 12 or more lymph nodes, is essential to staging and prognostication.
Current medical therapies for pancreatic neuroendocrine tumors are limited to the unresectable (T4) or metastatic disease, with no demonstrated benefit for adjuvant therapy after surgical resection. While somatostatin analog therapy is established for midgut NET [46] improving progression-free survival, it is not effective for PNETs [47, 48]. Cytotoxic chemotherapies, classically streptozocin, fluorouracil, and doxorubicin or more recently temozolimide and capecitabine combinations, have traditionally been the mainstay of treatment for advanced PNETs. In 2011, two randomized controlled trials of biologic therapies were published which changed the chemotherapeutic landscape for NETs. Use of sunitinib (a multitargeted receptor tyrosine kinase inhibitor) was shown to effect more than a doubling in progression-free survival (11.4 months vs. 5.5 months, P < 0.001) and an increase in overall survival. Similarly, the mammalian target of rapamycin (mTOR) inhibitor everolimus more than doubled the progression-free survival from 4.6 months to 11.0 months [49, 50]. While PNETs can present with advanced disease, the natural history of disease is rather indolent, laying the groundwork for innovative therapies for this challenging disease.
Hepatic resection for liver metastases where complete resection, or at least greater than 90 % cytoreduction, can be achieved is advocated in selected patients with metastatic PNETs. In addition, locally ablative therapies have been utilized to treat hepatic metastases with clinical success. Liver transplantation is undertaken for metastatic PNETs only in highly selective cases with very favorable tumor biology (low mitotic count, low Ki-67 index, well-differentiated histology) where all other medical therapies have failed.
Midgut
Midgut carcinoids include those found in the jejunum, ileum, appendix, cecum, and proximal colon. Histologically, midgut carcinoids appear as a solid mass of cells and stain with argentaffin silver. Secretory products from midgut carcinoids include serotonin, prostaglandins, and polypeptides. Hypersecretion of serotonin and kallikrein, in particular, are known to lead to carcinoid syndrome if liver function is decreased and these hormonal products are not cleared from the portal system and circulate systemically, usually because of carcinoid metastases to the liver. Elevated levels of 5-HIAA, a metabolite of serotonin, in the urine is diagnostic for carcinoid syndrome. The potential of liver metastases among midgut carcinoids depends on location, ranging from 2 % (appendix), to 35 % (small bowel), to 60 % (ascending colon).
Small Bowel (Jejunum, Ileum)
While NETs are rare types of neoplasms in other parts of the GI tract (i.e., 3 % in the pancreas), NETs make up one-third of all small bowel neoplasms. They are almost always found within 60 cm of the ileocecal valve in the ileum, arising from serotonin-secreting enterochromafin cells [51]. They can metastasize to lymph nodes early, despite a small size. Patients presenting with small bowel carcinoids are usually in their 6th or 7th decade of life and present with abdominal pain and/or small bowel obstruction [52].
Prognosis for patients with small bowel carcinoids depends on a number of factors. Age >65, tumor size >2 cm, and depth of invasion beyond the muscularis propria are predictors of poor prognosis [53]. Surgical resection is indicated for primary carcinoids of the small bowel, with en bloc lymphadenectomy. If the tumor has metastasized or if carcinoid syndrome is evident, preoperative and intraoperative octreotide treatment is indicated. Octreotide administration prevents carcinoid crisis (extreme hypotensive episode) during surgical resection by mitigating the effects of hormonal release [54]. Postoperatively, octreotide long-acting repeatable (LAR) is indicated in patients with metastatic disease [55]. The presence of metastatic disease is a primary determinant of long-term prognosis, as 5 years overall survival without metastatic disease approaches 60 %, but is as low as 30 % in the presence of liver metastases [56].
Appendix
Incidence of appendiceal carcinoids ranges from 16 to 45 % of GI carcinoids [57]. The majority are asymptomatic and are located in the distal 1/3 of the appendix. Metastases are rare, especially with small tumors <2 cm, so carcinoid syndrome occurs in only 10 % of cases. A large tumor or tumor located in the proximal appendix may be symptomatic, leading to carcinoid syndrome, appendicitis, and/or possible bowel obstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree