Fig. 9.1
Symptomless neck mass
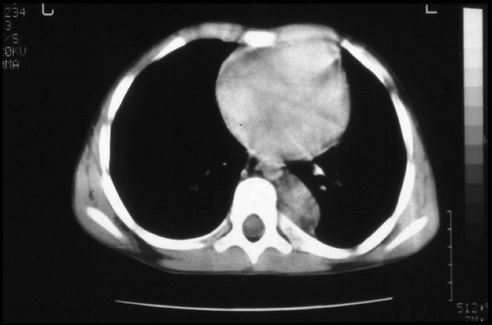
Fig. 9.2
Incidental finding on chest X-ray
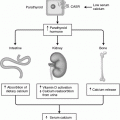
The abdomen accounts for 60% of the primary tumours half of these arise in the suprarenal gland. A further 15% arise in the thorax; 10% in the pelvis and the remainder in other sites.
Horner syndrome may arise when a tumour develops in the cervical sympathetic chain and is usually permanent. Paralysis from spinal cord compression is a risk with intra spinal disease and is a therapeutic emergency. Paraneoplastic syndromes such as watery diarrhoea and hypertension are manifestations of tumour endocrine secretion while the explanation for the disabling Opsoclonus Myoclonus or dancing eye syndrome is not known.
Investigation
The first step is an appropriate suspicion. Listen to the parents, examine the child and if no obvious cause for illness is found then consider a readily available and non-invasive ultrasound scan.
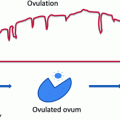
If suspicion is confirmed, the minimum diagnostic set includes urine catecholamine assay, cross-sectional imaging and biopsy of tumour and bone marrow. The aim is to obtain a tissue diagnosis and detect metastatic disease. Ultrasound scan (US) is often the first investigation but Computed Tomography (CT) (Fig. 9.3) or Magnetic Resonance imaging (MR) are mandatory. Tissue diagnosis will require US or CT guided needle core biopsies of the primary tumour, sometimes aided by endoscopy. Multiple cores are necessary [4–9] to allow biological as well as histopathological analysis. Discrete tumours may be excised at presentation if this is deemed safe on imaging. Either way the tissue must be sent fresh to the attending pathologist and without added preservative. The majority of tumours will take up meta-iodo-benzyl guanidine (MIBG) (Fig. 9.4), a radio-labelled precursor of noradrenaline. Most treatment protocols now include MIBG gamma scanning to detect metastatic disease.
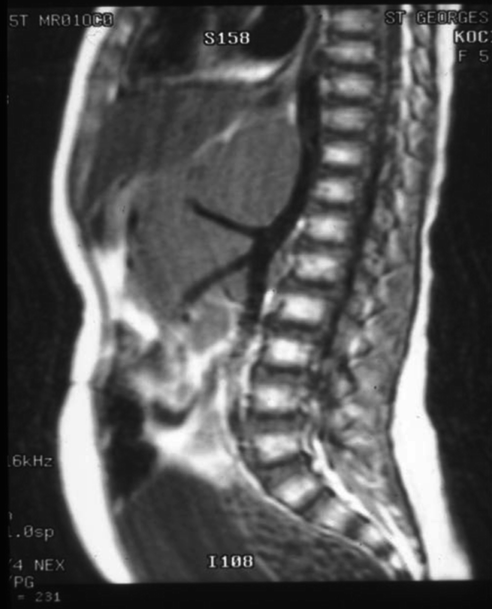
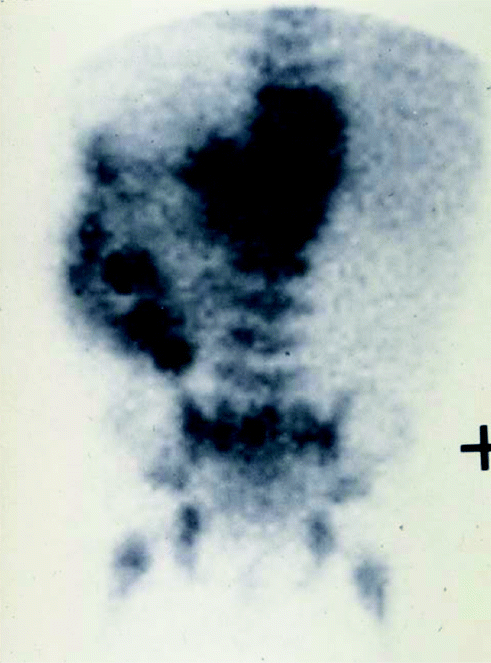

Fig. 9.3
Tendency to surround major vessels

Fig. 9.4
MIBG of neuroblastoma
Treatment History
Unsurprisingly early treatment was surgical. The first recorded operation for neuroblastoma was by Bartlett in 1916 [4, 10]. The operation was a success and the patient still alive 15 years later Subsequent reports were very pessimistic with no survivors in a 1934 report [5] Prior to 1937, there were only 2 of 20 survivors without operation [6].
The addition of radiation therapy improved survival: with 21 survivors out of 24 patients in 1959, most of whom received radiation therapy after tumour excision [7]. In a similar study, the addition of radiation therapy improved survival from one of nine to six of seven following incomplete excision [8]. Radiation therapy alone resulted in five survivors [9] but all were infants, now known to have a good prognosis by virtue of their age.
Chemotherapy, now the mainstay of treatment for advanced disease was slow to gain acceptance. The Subcommittee on Childhood Solid Tumors of the National Cancer Institute USA in 1970 found no significant difference in survival when a cohort of patients treated in 1956 were compared with a similar cohort treated in 1962. These eras were before and after the use of actinomycin D, cyclophosphamide and vincristine [11].
Staging
Audrey Evans and others realised that there were patient and tumour characteristics which would predict survival independent of therapy. A disease staging system was proposed by the CCSGA [15] (Table 9.1) which led the way to disease risk stratification. The prognostic significance of patient age and disease stage was confirmed by analysis of the outcome of patients from CCSG and Children’s Hospital of Philadelphia [16]. The staging principles were developed further by a larger group and published as the International Neuroblastoma Staging System (INSS) [17, 18] (Table 9.2). The distribution of INSS stages is shown in (Fig. 9.5).
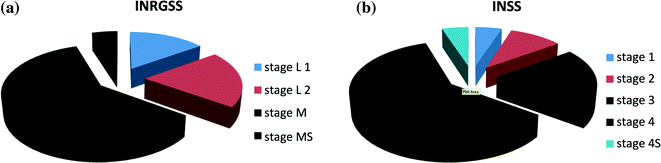
Table 9.1
CCSG staging
Stage I | Tumour confined to the organ or structure of origin |
Stage II | Tumour extending in continuity beyond the organ or structure of origin but not crossing the midline. Regional lymph nodes on the ipsilateral side may be involved |
Stage III | Tumour extending in continuity beyond the midline. Regional lymph nodes may be involved bilaterally |
Stage IV | Remote disease involving the skeleton, organs, soft tissue and distant lymph node groups |
Stage IV-S | (Special category). Patients who would otherwise be Stage I or II but who have remote disease confined to liver, skin or bone marrow and who have no radiographic evidence of bone metastases on complete skeletal survey |
Table 9.2
International staging system for neuroblastoma
Stage 1 | Localised tumour confined to the area of origin; complete gross excision, with or without microscopic residual disease; identifiable ipsilateral and contralateral lymph nodes negative macroscopically |
Stage 2a | Unilateral tumour with incomplete gross excision; identifiable ipsilateral and contralateral lymph nodes negative microscopically |
Stage 2b | Unilateral tumour with complete or incomplete gross excision; with positive ipsilateral regional lymph nodes; contralateral lymph nodes negative microscopically |
Stage 3 | Tumour infiltrating across the midline with or without regional lymph node involvement; or, unilateral tumour with contralateral regional lymph node involvement; or, midline tumour with bilateral regional lymph node involvement |
Stage 4 | Dissemination of tumour to distal lymph nodes, bone, bone marrow, liver and/or other organs (except as defined in Stage 4S) |
Stage 4S | Localised primary tumours defined for Stage 1 or 2 with dissemination limited to liver, skin and/or bone marrow |

Fig. 9.5
a and b Stage distribution INRGSS with INSS
The latest and arguably the most thoroughly researched staging system was developed by The International Neuroblastoma Risk Group (INRG) [19] and based on data from almost 9000 patients. The INRG Staging System (INRGSS) [20] was derived from an earlier study which demonstrated the importance of Surgical Risk Factors (SRF) in the prediction of the risk and effectiveness of operations to excise the tumour [21]. In essence a SRF is present when a vital structure, typically a large blood vessel, is encased by tumour [22]. The INRGSS, as it has its basis on pre-operative imaging overcomes some of the subjectivity of INSS which in part was based on operative outcome. A comparison of the distribution of stages in INSS and INRGSS is shown in (Fig. 9.5a, b). For consistency INRGSS, is used in this text except when citing a reference in which case the contemporary staging system is respected.
Pathology
The heterogeneity in behaviour of neuroblastic tumours is reflected by a wide spectrum in their microscopic morphology. The spectrum ranges from tumours with well differentiated cells which closely resemble mature neural elements to those comprised of undifferentiated small round blue cells, morphologically indistinguishable from other malignant childhood tumours and resembling undifferentiated embryonic blast cells (Fig. 9.6a, b).
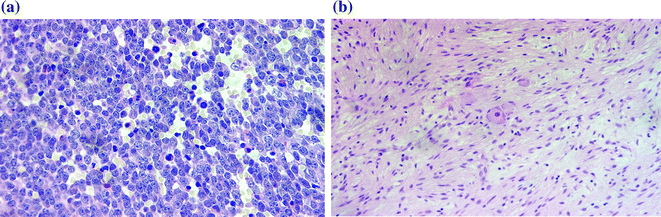

Fig. 9.6
a Undifferentiated cells. b Differentiated cells
The histological appearance has a profound influence on tumour behaviour [23–25]. Key variables are the degree of differentiation of the neuroblasts and the predominance of mature Schwann cells or stroma.
The International Neuroblastoma Pathology Committee (INPC) formed in 1994 with the aim of standardising histopathological definitions and tumour morphology. The Committee refined the classification as more information became available [26–28]. In ascending order of malignancy and from differentiated to undifferentiated, there are three tumour types: ganglioneuroma; ganglioneuroblastoma; and neuroblastoma.
Molecular Pathology
Extensive studies at a sub-cellular level have added further precision to the prediction of tumour behaviour. One of the first variables to emerge was DNA ploidy: tumours with diploid DNA have a worse prognosis than those with hyperdiploid [29]. One of the first genetic predictors of tumour behaviour to be identified was amplification of the MYCN oncogene [30] (Fig. 9.7a, b), which was associated with an unfavourable prognosis. Chromosomal aberrations associated with unfavourable prognosis, include 1p deletion [31]; loss of chromosome 11q [32]; gain of chromosome arm 17q [33]. Oncogenic mutations of ALK (anaplastic lymphoma kinase) are also associated with poor prognosis [34]. An elegant multivariate analysis of the impact of all these genetic variables on patient survival by Wendy London may be found in the INRG task force report [19].
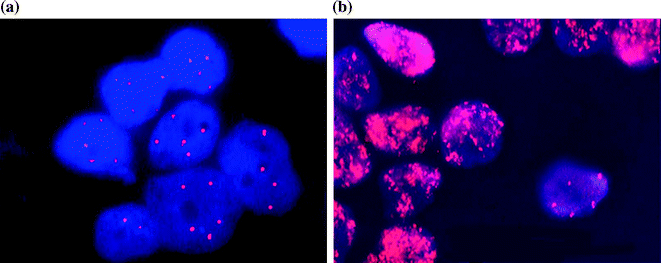

Fig. 9.7
a and b MYCN amplification
Risk-Based Therapy
As overall survival improved from around 20% in 1970 to around 50% in 1990 [35, 36] it became clear that the risk of disease could be estimated by clinical and biological factors including: patient age, tumour stage, histology and MYCN status [37–39]. This information allowed the many effective therapies to be matched with the needs of the patient and the trend was for less therapy rather than more.
Patients with metastatic disease had the worst outcome and required intensive multimodal therapy while the prognosis for patients with localised disease was much better [40, 41]. Indeed some patients with localised disease, were cured by operation alone [42–44], with the implication that operation was the least toxic therapy for patients with localised disease.
The trend towards less intervention was extended by the German study group (GPOH) who observed spontaneous regression in 44 of 93 infants who did not receive any treatment for their localised disease [45].
Predicting the Risk of Operation
The trend towards operation as the sole treatment for localised disease demanded that the risk of surgery be predicted with as much accuracy as possible. This was emphasised by reports of serious surgical complications and even post-operative deaths in this generally good prognosis group of patients [46, 47].
In 1995, the European Neuroblastoma Study Group opened a study to examine the risk of operation based on imaging before surgery (LNESG1). Data were collected from 107 institutions in 10 European countries. Cross-sectional imaging studies from each patient were evaluated to detect features which would predict the probability of complete tumour resection and the risk of operation complication. Surgical Risk Factors (SRF) were defined for each tumour site based on the risk of damage to neighbouring structures [21]. These risks arose as a result of the tendency for Neuroblastoma to encase vital structures, in particular major blood vessels (Fig. 9.8a and b).
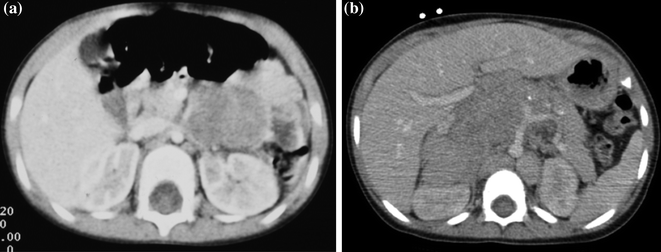

Fig. 9.8
a Cross-sectional imaging showing absence of risk factors (SRF/IDRF). b Cross-sectional imaging showing presence of risk factors
The presence of SRF was associated with a complete excision rate of 46%, compared with 75% when SRF were not present. The operative complication rate was 17% if SRF were present compared with 5% in the absence of SRF.
This study was the first to estimate the variability in the complexity of operation and predict its risk and efficacy. The implication from this study was that patients with tumours which exhibit SRF should have neo-adjuvant therapy before operation in anticipation of a reduction in tumour volume and vascularity and thus a safer operation.
The tumour in patients with advanced disease INRGSS L2 and M almost invariably exhibits vascular encasement with SRF/IDRF. In spite of very effective chemotherapy, these risk factors rarely disappear completely and the surgeon is faced with technical hazards which would normally discourage operation. In spite of this, most contemporary treatment protocols include a determined attempt to remove the entire tumour at operation following more or less intensive chemotherapy.
Even in these patients with advanced disease, who may be regarded as the most challenging for the surgeon, the often lengthy operation may be accomplished with low morbidity and mortality (Fig. 9.9). The death rate as a result of operation is less than 1% and the complication rate less than 10% [48–50]. The operative techniques were elegantly described by Kiely. For abdominal tumours, the overlying viscera must be dissected and reflected to expose the posterior abdominal wall. Dissection then proceeds along the major vessels starting at a section free from tumour and dissecting the latter from the vessels. Only in this way may branches and tributaries be identified and preserved.
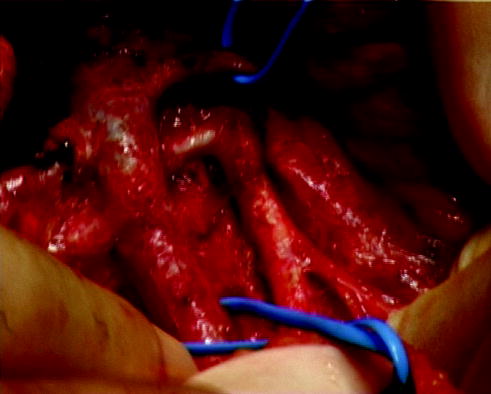

Fig. 9.9
Operation showing clearance of advanced neuroblastoma from major abdominal vessels
The scope of minimally invasive surgery (MIS) for Neuroblastoma is currently under review. Smaller and localised tumours are already safely and effectively excised by MIS. It is likely that MIS will prove effective with more extensive tumours in future always with the proviso that oncological principles are respected.
Predicting the Risk of Disease
The variability in behaviour of neuroblastoma and its relation to clinical and biological variables became increasingly apparent [40, 41]. The International Neuroblastoma Risk Group (INRG) sought to develop a consensus for risk stratification before treatment [19]. In a large part, this was driven by the need for a system which would gain world-wide acceptance and thus facilitate accurate comparison of risk-based outcome studies from any national or international study group.
Data were collected from 8800 comparable patients and 13 variables analysed for prognostic significance. The most important and discriminatory factors were tumour stage, patient age, histology grade, MYCN and 11q status and DNA ploidy. Four risk groups emerged with 5 year event free survival (EFS) of: >85; >75; >50 and <50%. The INRG also introduced a novel staging system (INRGSS) again based on patient and tumour characteristics before treatment [20]. In summary, the patient stages were defined by Image Defined Risk Factors (IDRF), equivalent to SRF and based on pre-operative cross-sectional imaging. The stages were L1, a localised tumour with no IDRF; L2, a localised tumour with IDRF; M, a tumour with distant metastases and MS, a small primary tumour with metastases confined to skin and liver and in a patient less than 18 months of age. Emphasis was placed on staging before operation. In this way, the outcome of operation as a staging factor was eliminated. This cornerstone of INSS was considered an imprecise variable as it was dependent on the persistence and or skill of the surgeon and the outcome of operation.
Treatment Strategies
Neuroblastoma cells are susceptible to many therapeutic agents and sometimes undergo apoptosis. There are now strong data which allow the risk of the disease to be predicted with accuracy. For many years, a main driver of clinical research has been matching the risk of treatment with the risk of disease. For these reasons, treatment strategies will be presented in relation to the category of disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree