© Springer Science+Business Media New York 2016
Marina Kurian, Bruce M. Wolfe and Sayeed Ikramuddin (eds.)Metabolic Syndrome and Diabetes10.1007/978-1-4939-3220-7_1717. Neural Modulation in the Treatment of Obesity
(1)
Department of Surgery, Mayo Clinic, 200 1st Street SW, Rochester, MN 55905, USA
Keywords
Peripheral neural modulationObesityObesity epidemicBariatric proceduresBrain–gut axisGastric stimulationGastric PacingCentral neural modulationWith the obesity epidemic, multiple efforts have been directed at developing and evaluating new, novel, and innovative therapies to induce clinically relevant weight loss. The majority of bariatric procedures involve anatomic changes designed to restrict oral intake mechanically; these procedures are usually in conjunction with some other influences related to the neurohormonal sequelae of a duodenal bypass (Roux-en-Y gastric bypass) or the removal of the majority of the ghrelin-secreting tissue (sleeve gastrectomy). These procedures, however, require major surgery, and any less invasive, easily reversible intervention would generate tremendous interest if proven effective.
Recently, exploitation of neural modulation of physiologic function has become a viable option in several disciplines—cardiac, pulmonary, central nervous system, and, of course, gastrointestinal [1–5]. Research directed specifically at the brain–gut axis has had a long, renowned history in surgery—i.e., Dragsted’s work on the role of vagal innervation on acid secretion, gastric emptying, and other unanticipated/unexpected and as yet poorly understood effects of vagotomy on appetite. The latter effects have spurned a renewed interest in the field of obesity.
17.1 Vagal Control of Upper Gut Function
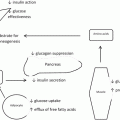
Physiologically , the vagus nerves mediate the neural axis of acid secretion from the stomach as well as modulate gastric motility/emptying [6] via proximal gastric receptive relaxation and an ongoing, incompletely understood, and inconsistent effect on antropyloric contractile activity exploited by the Dragstedian operative strategy of vagotomy and pyloroplasty. The vagus neural innervation also modulates pancreatic enzyme secretion involved in luminal digestion of ingested food. In addition, vagal afferent pathways to the central nervous system serve as sensors of gastric distention, luminal nutrients, pH, and even more poorly understood signals from the mid gut. Thus, the vagus nerves are an active, two-way highway by which the brain and the gut communicate constantly. Thus, why not attempt to exploit this brain–gut axis to induce weight loss?
Early on (1970s/1980s), there was an interest in utilizing a truncal vagotomy to augment the effects of the various gastric partitionings (so-called “gastric staplings”). Interest in vagotomy and appetite was based on observations in patients undergoing vagotomy for peptic ulcer disease. The scientific basis for this assumption of a vagal role in satiety was, however, absent. Results were minimal at best, poorly documented, and even more poorly conceived, and well-designed, controlled studies were absent. The secondary concern and laboratory support of “adaptation” to the effects of complete neural transection (i.e., other neural pathways or end organ changes) which negate the initial effects have dampened markedly the interest in operative vagotomy.
More recently, however, the appreciation of vagal afferent pathways involved in appetite [7, 8] and the feeling of satiation have refocused the concepts and interest in neural modulation in the treatment of obesity—i.e., gastric neural “stimulation” and “intermittent reversible vagal blockade” to be discussed below .
17.2 Gastric Stimulation/Pacing for Treatment of Obesity
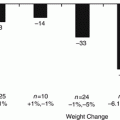
Early work by Cigaina from Venice working originally with pigs and especially with the pig “Lucky” in the early 1990s [9] generated tremendous interest in this form of neural modulation of eating, satiety, and ultimately weight loss. Cigaina studied the eating habits of pigs after electrodes were implanted within the musculature of the gastric wall and showed that these pigs consumed less food per day and lost weight when the electrodes were “stimulated” electrically [9, 10]. This work culminated in the first human study by Cigaina in five patients (1996–1998) with almost unbelievable success—mean percent body weight (% EBW) loss of 70 % without major dietary restrictions, no imposed changes in lifestyle, and no substantive side effects! Subsequent small groups of 10, 10, and 30 patients had less dramatic but clinically relevant mean weight losses of 20–40 % EBW. As of 2004, Cigaina had treated at total of 65 patients with this technique with, again, almost unbelievable success. Unfortunately, none of these “trials” had non-treated control patients.
As would be expected, this early, incredibly “attractive” concept spurned tremendous interest in the bariatric world, and even more so, when Cigaina suggested that an algorithm could better select patients most susceptible for this treatment. Several preliminary trials were initiated [11]—one in Europe, the Laparoscopic Obesity Stimulation Study (LOSS) of 65 patients at eight clinical sites [12] and another in the USA, the Dual-Lead Implantable Gastric Electrical Stimulation Trial (DIGEST) of 30 patients [13]. Early results in both trials were encouraging and suggested clinically relevant weight losses of 20–30 % EBW.
Early in this experience with these open-label, non-randomized, preliminary clinical trials, the “science” of gastric stimulation was investigated in more depth. Different algorithms of electrical stimulation (changes in pulse width, pulse frequency, duty cycle, etc.) as well as anatomic site of electrode placement were evaluated. It must be stated that this form of gastric stimulation is not gastric “pacing” as occurs with cardiac pacemakers. Although the human stomach does have an underlying cyclic myoelectric rhythm of 3 cycles/min generated by a pacemaker region in the body of the stomach that regulates gastric phasic contractile activity, the parameters of gastric stimulation used to treat obesity do not “entrain” or “pace” the contractile activity of the stomach. Indeed, the electrical parameters utilized include a frequency of 40 Hz (40/min), pulse width of 450 μs, and a “duty cycle” of 2 s on and 3 s off. Such an electrical input to the gastric wall is not necessarily designed to affect gastric function (gastric emptying), although some evidence suggests that this electrical algorithm may tend to decrease gastric empting by inducing proximal gastric relaxation, inducing stretch receptors, and thereby increasing the feeling of satiety and decreasing appetite. Others have suggested that this form of gastric stimulation induces central nervous system effects mediated through stimulation of vagal afferent nerves, leading to centrally mediated effects on appetite, changes in release of regulatory peptides in the brain, and even the potential for vagal blockade of both afferent signals from the gut and vagal (sympathetic?) efferent signals to the gut [8, 14]. Suffice it to say, no one really knows if or how this gastric stimulation works; nevertheless, global interest was tremendous. An excellent analysis of gastric electrical stimulation was published by the Medical Advisory Secretariat of the Ministry of Health and Long Term Care in Toronto, Canada, in 2006 [15]. The conclusion to date was that the efficacy for weight loss was as yet unproven, but the concept was attractive and potentially effective, albeit still experimental.
As with all open-label preliminary studies, proof of efficiency required carefully controlled trials with objective outcomes using accepted methodology. The Screened Health Assessment and Pacer Evaluation (SHAPE) trial was just such a well-designed, prospective, randomized, placebo-controlled, double-blind, multicenter study initiated in 2004 and reported in 2009. Shikora and colleagues from nine medical centers in the USA collaborated in this randomized and importantly double-blinded study of the efficacy of gastric stimulation therapy in severe (Classes II and III) obesity [16]. Based on prior non-controlled work, a so-called Baroscreen algorithm derived to select “ideal” candidates was used to screen 4800 potential candidates. Ultimately, 190 patients were selected; 87 % were women and overall mean age was 44 years. The gastric stimulation electrodes were bipolar electrodes pulled through a subserosal tunnel to ensure appropriate placement within the gastric wall and checked endoscopically. The stimulator device was positioned within the anterior abdominal wall.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree