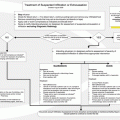
Prerenal azotemia
Volume depletion
Nausea, vomiting, diarrhea
Decreased oral intake owing to mucositis (5-fluorouracil, methotrexate, taxanes)
Polyuria caused by hyperglycemia (steroids) or diabetes insipidus (pituitary tumor)
“Third spacing” (hypoalbuminemia, liver or peritoneal metastases, interleukin-2)
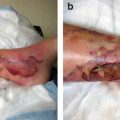
Insensible loss of fluid from skin lesions (mycosis fungoides)
Hemodynamic-mediated
Sepsis
Renal arteriolar vasoconstriction (NSAIDs, calcineurin inhibitors, hypercalcemia)
Congestive heart failure
Hepatorenal syndrome/hepatic sinusoidal obstruction syndrome
Budd-Chiari syndrome
Intrahepatic inferior vena cava compression or thrombosis caused by hepatomegaly or a tumor
IV iodinated contrast agent
Abdominal compartment syndrome
Intrinsic renal disease
Acute tubular necrosis
Chemotherapy (cisplatin, ifosfamide)
Anti-infectives (amphotericin B, foscarnet, cidofovir, aminoglycosides, vancomycin)
Bisphosphonates
Sepsis
Prolonged prerenal azotemia
Allergic interstitial nephritis (penicillins, cephalosporins, fluoroquinolones, NSAIDs)
Crystal nephropathy (methotrexate, acyclovir, ciprofloxacin, sulfonamides, rifampin)
Osmotic nephrosis (IV immunoglobulin, mannitol, starch)
Thrombotic microangiopathy (post-HSCT, gemcitabine, prior radiation therapy)
Myeloma-related kidney disease
Postrenal obstruction
Bladder outlet obstruction (malignancy of cervix, prostate, bladder, or uterus)
Retroperitoneal disease (metastasis, lymphadenopathy, fibrosis)
Hemorrhagic cystitis (cyclophosphamide, BK virus)
Ureteral strictures (prior radiation therapy, BK virus)
More than 35 different definitions of AKI are used in the literature, making cross-comparison of study results difficult. Recently, the Acute Dialysis Quality Initiative introduced the risk, injury, failure, loss, and end-stage renal disease (RIFLE) criteria for uniform classification of AKI (Table 6.2). The risk, injury, and failure categories define stages of AKI based on the percent increase in the level of serum creatinine relative to the baseline level or presence of oliguria. The loss and end-stage renal disease categories identify patients needing renal replacement therapy for more than 4 weeks and 3 months, respectively.
Table 6.2
RIFLE criteria for AKI
RIFLE stage | Increase in creatinine level | Decrease in urine output |
|---|---|---|
Risk | ≥50 % from baseline or 0.3 mg/dL | <0.5 mL/kg/h × 6 h |
Injury | ≥100 % from baseline | <0.5 mL/kg/h × 12 h |
Failure | ≥200 % from baseline or need for dialysis | <0.3 mL/kg/h × 24 h or anuria × 12 h |
Loss | Persistent AKI >4 weeks | |
End-stage renal disease | Loss of renal function >3 months | |
Researchers have validated the RIFLE criteria in numerous patient populations, and the criteria have proven useful in prognosis for AKI. For example, we found that at our institution, more than 12 % of patients with a baseline creatinine level less than 1.5 mg/dL upon admission to the intensive care unit had AKI. After multivariate analysis, the risk, injury, and failure categories of AKI were associated with increases in risk of death within 60 days after intensive care unit admission by 2.3-, 3.0-, and 14.0-fold, respectively. In patients with acute leukemia starting induction chemotherapy, the estimated mortality rate increased progressively within 8 weeks for the no-AKI, risk, injury, and failure categories (3.8 %, 13.6 %, 19.6 %, and 61.7 %, respectively). Small increases in serum creatinine level are closely associated with increased mortality rates and must be recognized early by physicians to prevent further deterioration of renal function . Therefore, identification and treatment of early-stage AKI (i.e., the risk category in the RIFLE criteria) at presentation is crucial.
Upon presentation at an emergency center, a thorough clinical examination and hemodynamic optimization are imperative for patients with AKI. Orthostatic hypotension, tachycardia, poor skin turgor, dry mucous membranes, and low central venous pressure suggest volume depletion, so intravenous (IV) hydration should be administered until the patient appears to be clinically euvolemic. A blood urea nitrogen:serum creatinine ratio greater than 20, fractional excretion of sodium less than 1 %, a urine sodium level less than 20 mEq/L, and the presence of hyaline casts in urinalysis suggest prerenal azotemia . Fractional excretion of sodium greater than 2 %, a urine sodium level greater than 40 mEq/L, and the presence of coarse granular casts in urinalysis are more suggestive of acute tubular necrosis . Urinary obstruction is generally indicated by hydronephrosis in ultrasonography, although hydronephrosis may not develop in patients with significant retroperitoneal disease. Patients with severe bladder outlet obstruction may have a palpable bladder. In diagnosis of urinary obstruction or retention, use of a portable bladder scanner may confirm an elevated postvoid residual urine volume (greater than 50–100 mL).
The optimal fluid-based therapy for AKI, especially in patients with sepsis, has been a subject of much debate. IV albumin may be given, but it has not proven to be more effective than crystalloid solutions. Other colloids, such as IV starch, are directly injurious to the kidney in that they cause osmotic nephrosis of the renal tubules; thus, in general, their use should be avoided in patients with AKI. In addition, albumin and starch leak out of the intravascular compartment within hours after administration, thereby potentially worsening peripheral edema. We generally prefer using crystalloid solutions such as isotonic saline (0.9 % saline) for volume resuscitation. Patients with sepsis have systemic vasodilation that contributes to hypotension and, likely, the need for vasopressor support. Continuous infusion of norepinephrine (2–12 μg/min) or vasopressin (0.01–0.04 U/min) is generally used to achieve a target mean arterial pressure of 70 mm Hg to preserve perfusion of vital organs. Placement of a Foley catheter should be attempted if the patient has signs of bladder outlet obstruction or urinary retention. Emergent placement of a percutaneous nephrostomy (PCN) tube may be necessary if the site of obstruction is above the level of the bladder outlet. Use of nephrotoxic medications and iodinated contrast agents should be avoided, if possible.
Currently, an effective therapy for AKI does not exist, and supportive dialysis may be necessary until AKI resolves. Some patients with AKI who present to an emergency center may urgently need dialysis. These are patients with uncontrollable hyperkalemia, extreme fluid overload, severe metabolic acidosis, uremia, or marked tumor lysis syndrome (TLS) . Early nephrology consultation in the emergency center would facilitate dialysis in these patients in a timely manner. Intermittent hemodialysis is generally sufficient for volume and metabolic clearance. However, patients with septic shock or severe TLS may need continuous renal replacement therapy. We have found that continuous, sustained low-efficiency dialysis in the intensive care unit provides optimal metabolic clearance and minimizes the cumulative positive fluid balance in patients who are hemodynamically unstable.
Multiple Myeloma and AKI
Multiple myeloma is a neoplastic disorder of plasma cells that results in the overproduction of monoclonal immunoglobulins and fragments (paraproteins). These paraproteins circulate in the bloodstream and may deposit in vital organs, leading to tissue injury. AKI is a common manifestation of paraprotein deposition in the kidneys. Classic myeloma cast nephropathy develops when paraproteins filter through the glomeruli and bind to Tamm-Horsfall mucoprotein in the distal tubule, leading to cast formation and tubular obstruction. Another manifestation is immunoglobulin light chain amyloidosis (primary amyloidosis), in which paraproteins undergo conformational changes and deposit as amyloid fibrils in the glomeruli and vasculature. Lastly, light chains may deposit within the glomerular and tubular basement membranes, leading to light chain deposition disease.
The clinical presentation of AKI in patients with multiple myeloma may be insidious, with only mild proteinuria or severe proteinuria with fulminant renal failure. More than half of all patients with multiple myeloma present with some degree of AKI, and 10 % of them need dialysis. Physicians must consider occult multiple myeloma in elderly patients who have acute or chronic kidney disease with no obvious etiology. Initial work-up for multiple myeloma consists of serum and urine protein electrophoresis to detect elevated levels of monoclonal proteins. Serum-free light chain assays are now widely available, and use of them has increased the sensitivity of detection of monoclonal proteins in serum. Routine qualitative dipstick urinalysis, which detects albuminuria, does not detect monoclonal proteins (Bence Jones proteins). Light chain deposition disease and amyloidosis cause glomerular damage, leading to significant albuminuria. In contrast, classic myeloma cast nephropathy spares the glomeruli, and patients with it typically present with only mild albuminuria. Patients with amyloidosis may also present with systemic manifestations such as restrictive cardiomyopathy, hepatomegaly, carpal tunnel syndrome, and orthostatic hypotension. Renal biopsy analysis reveals light chain or amyloid deposits, which provides the definitive diagnosis.
Patients who present with multiple myeloma and renal disease must undergo aggressive treatment to preserve kidney function. Initial hydration consists of infusion of normal saline, with a urine output goal of 2.5–3.0 L a day, which helps prevent the formation of casts. Therapy aimed at decreasing the production of paraproteins (i.e., steroids) should be instituted immediately to alleviate end-organ damage. Use of concomitant nephrotoxic medications reportedly potentiates renal injury. Use of aminoglycosides, IV contrast agents, diuretics, and nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided. Hypercalcemia commonly occurs in patients with multiple myeloma. Although the risk of acute tubular necrosis is low, therapy with a bisphosphonate should be considered if hypercalcemia does not resolve with the use of hydration, loop diuretics, and calcitonin (3–4 mg of zoledronic acid diluted in 100 mL of normal saline administered in an IV infusion for at least 15 min). A large randomized controlled trial of plasmapheresis in the setting of AKI secondary to myeloma did not demonstrate a significant improvement in the composite endpoint of death, dialysis dependence, and glomerular filtration rate less than 30 mL a minute. Use of high cutoff filters for rapid removal of light chains via hemodialysis may be more effective than plasmapheresis and is currently being studied in Europe.
Electrolyte Abnormalities
TLS
TLS can be a life-threatening emergency in patients with cancer. Massive tumor-cell breakdown releases potassium, phosphorus, and uric acid into the extracellular environment, which overwhelms the excretory capacity of the kidneys. Hyperkalemia may predispose patients to cardiac arrhythmias and sudden death. Hyperphosphatemia and secondary hypocalcemia may lead to muscular irritability, cardiac arrhythmias, and metastatic calcification. Uric acid may precipitate in the renal tubules and cause AKI. TLS generally occurs in patients receiving chemotherapy , although it can occur spontaneously.
Patients with rapidly proliferating hematologic malignancies are at the greatest risk for TLS. Risk factors for TLS include a white blood cell count greater than 50,000/μL, elevated lactate dehydrogenase level, bulky tumor, advanced age, and chronic kidney disease. Although patients with lymphoma or acute leukemia are at greatest risk, authors have reported cases of TLS in patients with a variety of solid tumors undergoing chemotherapy and/or radiation therapy.
Identification of TLS is fairly straightforward in a patient who presents with marked derangements in electrolyte levels. The diagnosis may be less clear in patients in whom AKI is secondary to an effective prerenal state, such as volume depletion or hypotension. Similar to patients with TLS, these patients may have hyperkalemia, hyperphosphatemia, and hyperuricemia. In the absence of other risk factors for TLS, these patients generally experience improvement in electrolyte levels and renal function with hydration or normalization of blood pressure.
Management of TLS largely consists of maintaining adequate urine output to facilitate excretion of potassium, phosphorus, and uric acid. Infusion of isotonic saline should be instituted 24 h prior to chemotherapy at 80–100 mL/m2 an hour and titrated accordingly to maintain a urine output of at least 2.5 L a day. Fluid management may be affected by underlying heart failure. Alkalinization of the urine with IV sodium bicarbonate may help prevent the formation of uric acid crystals but may increase the risk of calcium phosphate crystal deposition. Therefore, routine use of sodium bicarbonate in patients with TLS is no longer recommended.
Another important part of treatment of TLS is normalization of serum uric acid levels . Traditional treatment of hyperuricemia included daily administration of allopurinol (100–300 mg orally or intravenously) to decrease the production of uric acid. Unfortunately, allopurinol may be ineffective in patients with massive cell lysis. Rasburicase (0.2 mg/kg [IV] daily for up to 5 days) converts uric acid into readily excreted allantoin and was recently approved for the prevention and treatment of hyperuricemia. Serum uric acid levels often decrease until they become undetectable after rasburicase-based treatment. Whether this more pronounced hypouricemic effect of rasburicase than of allopurinol translates into improved renal and patient outcomes is unknown.
Patients with TLS who have electrocardiogram abnormalities , arrhythmias, or oliguria should be evaluated immediately by a nephrologist for renal replacement therapy. Use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and NSAIDs should be avoided because they may cause hyperkalemia and reduce glomerular filtration . Prophylactic dialysis is not recommended for patients with TLS or isolated hyperuricemia in the absence of AKI.
Hyponatremia
Authors have reported hyponatremia in 3.8 % of patients in emergency centers, although its incidence in cancer patients presenting to emergency centers is not known. The reported frequency of hyponatremia in patients admitted to hospitals based on a serum sodium level less than 135 mEq/L varies widely, ranging from 5.5 % to 28.0 %. In our institution, we found that 48 % of hospitalized patients had serum sodium levels less than 135 mEq/L. Increased hyponatremia severity was closely associated with increased length of hospital stay and 90-day mortality rate. The most common causes of hyponatremia in patients with cancer are hypovolemia and syndrome of inappropriate antidiuretic hormone secretion (SIADH) . Common etiologies of SIADH include malignancy (e.g., lung, gastrointestinal, central nervous system), pneumonia, use of certain drugs (e.g., antidepressants, haloperidol, carbamazepine, cyclophosphamide, vincristine), nausea, and pain. Renal salt wasting resulting from chemotherapy, “tea and toast syndrome ” resulting from malnutrition, and adrenal insufficiency resulting from metastasis or steroid withdrawal should be considered when assessing patients with hyponatremia.
Patients with hyponatremia may present with mild symptoms such as confusion, dizziness, nausea, and lethargy. Severe symptoms include seizures, coma, and death. The occurrence of symptoms of hyponatremia depends primarily on the rate of decline of the serum sodium level as opposed to its actual measured level. The brain is able to adapt to hyponatremia by excreting osmolytes from cells to prevent cerebral edema. If the rate of decline in the serum sodium level outpaces the excretion of osmolytes, cerebral edema with eventual herniation of the brain stem may develop. This requires immediate treatment to raise the serum sodium level until the patient is asymptomatic. If the decline in serum sodium level is more gradual, the patient may be asymptomatic or have only mild symptoms. Immediate treatment is not indicated in this situation.
Initial work-up for hyponatremia should include a physical examination to assess the patient’s volume status, chemistry profile, plasma osmolality, urine electrolyte levels, and urine osmolality. Patients with volume depletion have urine sodium levels less than 20 mEq/L and concentrated urine (urine osmolality greater than plasma osmolality). Patients with hypervolemia (those with heart failure, cirrhosis, third spacing caused by peritoneal or liver metastases, hypoalbuminemia, or inferior vena cava compression or obstruction) have signs of fluid overload upon physical examination (e.g., edema, ascites, effusions) but are in an effectively prerenal state. Therefore, they will also have urine sodium levels less than 20 mEq/L and concentrated urine. Patients with SIADH have urine sodium levels greater than 40 mEq/L and inappropriately diluted urine (urine osmolality less than plasma osmolality). Finally, patients with tea and toast syndrome or malnutrition have serum sodium levels less than 20 mEq/L along with diluted urine.
Hyponatremic patients who are asymptomatic or have mild symptoms generally do not need immediate treatment. Rapid correction of hyponatremia in these patients will increase the risk of osmotic demyelination syndrome . If patients have volume depletion, they should receive isotonic fluids such as normal saline. Otherwise, fluid administration should be restricted to less than 1 L a day. Salt tablets may be given to patients without hypervolemia (initially, 1 g 3 times a day). The introduction of vasopressin receptor antagonists revolutionized treatment of hyponatremia in patients with hypervolemia or SIADH. These drugs block the effect of antidiuretic hormone on the collecting ducts of the kidney, thereby stimulating water diuresis. Oral tolvaptan (7.5–15.0 mg daily) or IV conivaptan (20-mg loading dose with 20 mg administered over the ensuing 24 h) may be given with close monitoring of the sodium correction rate.
Treatment of hyponatremia in patients with severe symptoms consists of infusion of 3 % saline at a rate of 0.6–1.0 mL/kg an hour and initial monitoring of serum sodium levels at least every 2–4 h. The infusion is continued until the sodium level is greater than 120 mEq/L, symptoms have resolved, or the rate of sodium level correction exceeds 8 mEq within 24 h. Rates of correction in excess of 10–12 mEq per 24 h have been associated with osmotic demyelination syndrome , which may result in altered mental status, quadriparesis, quadriplegia, pseudobulbar palsy, coma, or death. Therefore, vigilant monitoring of serial sodium levels with titration of the 3 % saline infusion to prevent overcorrection of hyponatremia is necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree