The purpose of this article is to update the medical community on the current management of patients with appendiceal neoplasms. The authors discuss clinical evidence of medical and surgical treatment with emphasis on presentation, diagnosis, pathology, and surgical technique. Current available clinical evidence on the use of systemic chemotherapy is included. The authors describe in detail management of peritoneal carcinomatosis arising from tumors of the appendix with cytoreductive surgery and hyperthermic intraperitoneal therapy as standard of care.
Key Points
- •
Appendix tumors are rare and biologically diverse.
- •
Early referral after appendectomy results in improved outcomes.
- •
When confined to the abdominal cavity, peritoneal carcinomatosis from appendiceal origin is effectively treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC).
- •
Lymph node involvement is associated with poor prognosis.
- •
Patients with peritoneal carcinomatosis from appendiceal tumors should be referred and treated at a specialized center.
Introduction
Appendiceal cancers are found in less than 1% of appendectomy specimens. A population-based study from the Surveillance, Epidemiology and End Results program (SEER), from 1973 to 1998, reported the incidence of cancer of the appendix was 0.12 cases per 100,000 people per year and that the most common histology was mucinous adenocarcinoma.
Neoplasms of the appendix are not often suspected before surgery and are found either intraoperatively or on pathologic examination. The increasing awareness of the disease and its pathophysiology and presentation has sparked an increased interest in the surgical and medical oncology fields with respect to the treatment of diseases with peritoneal dissemination. The first reference to carcinoma of the appendix was a case reported by Merling in 1838. In 1903 Elting reported a review and case series from 1838 to 1903. Forty-three cases of neoplasms of the appendix were reported of which only 23 were true carcinoma of the appendix. Awareness of neoplasms of the appendix is increasing. They were previously diagnosed as mucinous neoplasms of the ovary. Recent immunohistochemical, molecular, and genetic evidence support an origin in the appendix in most cases with secondary involvement of the peritoneum and/or ovaries. More recently, the classification of the appendiceal carcinomas has been separated from the colorectal tumors in the seventh edition of the American Joint Committee on Cancer Cancer Staging Manual .
The appendix first appears at the eighth week of gestation as an out-pouching of the cecum and gradually rotates to a more medial location. Its length varies from 2 cm to 15 cm and is located at the convergence of the taeniae along inferior aspect of the cecum. The tip of the appendix is most commonly retrocecal, but it is pelvic in 30% and retroperitoneal in 7% of the population. The lymphatic drainage is into the anterior ileocolic lymph nodes and histologic examination shows goblet cells that are scattered throughout the mucosa.
An analysis from the SEER 1973 to 2004 database of appendiceal cancers (n = 2791) showed that adenocarcinoma accounted for 65.4% of appendiceal cancers, followed by neuroendocrine neoplasms (0.1%–0.2% over 30 years). The incidence of neuroendocrine neoplasms seemed stable whereas that of adenocarcinoma increased 2.6-fold during that time. The overall 5-year survival of appendiceal adenocarcinomas was reported in the SEER database as 46.2%. The reported 5-year survival for subgroups was adenocarcinoma 47.9%, mucinous adenocarcinoma 47.7%, mucinous cystadenocarcinoma 59.0%, signet ring cell carcinoma 20.3%, and lymphomas were 1.7%.
Introduction
Appendiceal cancers are found in less than 1% of appendectomy specimens. A population-based study from the Surveillance, Epidemiology and End Results program (SEER), from 1973 to 1998, reported the incidence of cancer of the appendix was 0.12 cases per 100,000 people per year and that the most common histology was mucinous adenocarcinoma.
Neoplasms of the appendix are not often suspected before surgery and are found either intraoperatively or on pathologic examination. The increasing awareness of the disease and its pathophysiology and presentation has sparked an increased interest in the surgical and medical oncology fields with respect to the treatment of diseases with peritoneal dissemination. The first reference to carcinoma of the appendix was a case reported by Merling in 1838. In 1903 Elting reported a review and case series from 1838 to 1903. Forty-three cases of neoplasms of the appendix were reported of which only 23 were true carcinoma of the appendix. Awareness of neoplasms of the appendix is increasing. They were previously diagnosed as mucinous neoplasms of the ovary. Recent immunohistochemical, molecular, and genetic evidence support an origin in the appendix in most cases with secondary involvement of the peritoneum and/or ovaries. More recently, the classification of the appendiceal carcinomas has been separated from the colorectal tumors in the seventh edition of the American Joint Committee on Cancer Cancer Staging Manual .
The appendix first appears at the eighth week of gestation as an out-pouching of the cecum and gradually rotates to a more medial location. Its length varies from 2 cm to 15 cm and is located at the convergence of the taeniae along inferior aspect of the cecum. The tip of the appendix is most commonly retrocecal, but it is pelvic in 30% and retroperitoneal in 7% of the population. The lymphatic drainage is into the anterior ileocolic lymph nodes and histologic examination shows goblet cells that are scattered throughout the mucosa.
An analysis from the SEER 1973 to 2004 database of appendiceal cancers (n = 2791) showed that adenocarcinoma accounted for 65.4% of appendiceal cancers, followed by neuroendocrine neoplasms (0.1%–0.2% over 30 years). The incidence of neuroendocrine neoplasms seemed stable whereas that of adenocarcinoma increased 2.6-fold during that time. The overall 5-year survival of appendiceal adenocarcinomas was reported in the SEER database as 46.2%. The reported 5-year survival for subgroups was adenocarcinoma 47.9%, mucinous adenocarcinoma 47.7%, mucinous cystadenocarcinoma 59.0%, signet ring cell carcinoma 20.3%, and lymphomas were 1.7%.
Pathology
Appendiceal tumors can be broadly classified as epithelial and nonepithelial tumors.
Epithelial Tumors
There are many existing classifications of epithelial appendiceal neoplasms and this reflects the lack of consensus among the pathologists. The limitations of all classification systems are well recognized and even a benign-appearing tumor may exhibit aggressive clinical course.
In 1940, Woodruff and McDonald classified cystic mucinous tumors of the appendix as mucoceles and cystadenocarcinoma grade 1 but by the 1960s to 1970s they were reclassified as mucinous cystadenomas or villous adenomas of the appendix. Higa and colleagues in 1973 classified appendiceal mucinous tumors as cystadenocarcinomas if associated with pseudomyxoma peritonei and cystadenomas if not. Over the past decades there has been controversy among pathologists regarding the classification of some appendiceal tumors due to lack of consensus on the invasive potential of the appendiceal epithelial cells. Some pathologists require destructive invasion of the appendix with infiltrating glands to make the diagnosis of adenocarcinoma and others require the presence of a broad front with neoplastic epithelium directly abutting the hyalinized cyst wall thinning out the muscularis mucosae.
In 1995, Carr and colleagues reviewed 184 tumors at the Armed Forces Institute of Pathology and proposed the following classification:
- 1.
Adenoma: dysplastic epithelium with mucin dissecting into wall with intact muscularis mucosae
- 2.
Mucinous tumors of uncertain malignant potential: well-differentiated mucinous epithelium without invasion or with mucin in the wall or outside the appendix with loss of muscularis mucosae
- 3.
Adenocarcinoma: invasive neoplastic cells beyond muscularis mucosae
In 2003 Misdraji and colleagues classified them as low-grade mucinous neoplasms and mucinous adenocarcinoma.
Pai and Longacre in 2005 also proposed a classification:
- 1.
Adenoma: mild-to-moderate atypia, mitosis, no stromal invasion, perforation with mucin
- 2.
Mucinous tumor of uncertain potential: adenoma with positive margin, mucin present within the wall
- 3.
Mucinous tumor–low malignant potential: adenoma with neoplastic cells in peritoneum
- 4.
Adenocarcinoma: invasive mucinous tumor
In 1995, Ronnett and colleagues analyzed the clinicopathologic features of 109 cases of multifocal peritoneal mucinous tumors and classified these as
- 1.
Diffuse peritoneal adenomucinosis (DPAM): mucin with fibrosis and scant simple to focally proliferative mucinous epithelium with minimal cytologic atypia and mitotic figures. The primary appendiceal tumor was an adenoma in all these cases.
- 2.
Peritoneal mucinous carcinoma (PMCA): the primary tumor is appendiceal adenocarcinoma with peritoneal tumors having more proliferative epithelium with signet ring cells and marked cytologic atypia.
There were 14 of 109 cases that were classified as PMCA–I (intermediate) because they showed features of DPAM with carcinoma in the peritoneal lesions, whether or not the primary site demonstrated carcinoma. In a follow-up study, Ronnett clarified that PMCA–I should be included into the PMCA group because they behaved similarly.
The authors’ group uses the modified Ronnett classification for peritoneal dissemination of appendiceal neoplasms. Its advantage is that it approximately divides the tumors into less-aggressive DPAM and more-aggressive PMCA, the latter having a potential to develop nodal, liver, and other metastases.
Every team specializing in treatment of appendiceal malignancies should establish a clear communication with a pathologist to have a common language when classifying the appendix tumors. Also, a critical review of the oncological outcomes should be conducted periodically to realign the pathologic classification used and the clinical practice. Further efforts should be undertaken by the collaboration of all centers treating these conditions to try to standardize the pathology of appendiceal neoplasms.
Nonepithelial Tumors
- 1.
Endocrine tumors
- a.
Classic appendiceal endocrine tumors
- b.
Goblet cell carcinomas
- a.
- 2.
Lymphoma
- 3.
Sarcoma
Endocrine tumors are classified according to the World Health Organization and TNM classifications.
WHO classification
- a.
Well-differentiated endocrine tumor (benign behavior and uncertain behavior)
- b.
Well-differentiated endocrine carcinoma, low grade, malignant
- c.
Mixed exocrine-endocrine, malignant, goblet cell carcinoid (GCC)
A TNM classification and grading scheme was proposed by the European Neuroendocrine Tumor Society in 2007 ( Table 1 ).
a 10 high power field (HPF) = 2 mm 2 , at least 40 fields (at 40 × magnification) evaluated in areas of highest mitotic density.
b MIB1 antibody; % of 2000 tumor cells in areas of highest nuclear labeling.
GCC tumors of appendix are rare endocrine tumors that have various names, such as adenocarcinoid, mucinous carcinoid, crypt cell carcinoma, and mucin-producing neuroendocrine tumor, but first coined, goblet cell carcinoid , in 1974 by Subbuswamy and colleagues. Current understanding of GCC’s origin states that it is an amphicrine tumor, which originates from a single undifferentiated pluripotent intestinal epithelial crypt base progenitor stem cell that has dual neuroendocrine and mucinous differentiation. The natural history of these tumors is intermediate between carcinoids and classical adenocarcinomas of the appendix ; hence, a proposed name is mucin-producing neuroendocrine tumor (or carcinoma) of the appendix. Unlike adenocarcinomas, K-ras and β-catenin expression is absent in these tumors. These tumors show allelic loss of chromosomes 11q, 16q, and 18q, similar to ileal carcinoids.
Classification
Group A: typical low-grade GCC
Group B: adenocarcinoma ex GCC with signet ring cell type
Group C: adenocarcinomas ex GCC, poorly differentiated
Clinical presentation of nonepithelial and epithelial neoplasms of the appendix
Carcinoids
Carcinoids are most commonly located at the tip of the appendix and they present most of the time with appendicitis. They are divided into 2 types. The insular type resembles enterochromaffin cell and produces serotonin. Lymph node and liver metastasis are rare. The tubular variant of carcinoid arises from the L-cell, which produces enteroglucagons and peptide YY. Immunohistochemistry can distinguish an adenocarcinoma from a tubular carcinoid because the latter is positive for chromogranin and/or synaptophysin. Tumors less than or equal to 1 cm require only an appendectomy. If 1 cm to 2 cm in size without involvement of the base of appendix, they are managed with appendectomy and the question of adding a right hemicolectomy depends on grade, mitotic activity, invasion of mesoappendix, or lymphovascular invasion. These patients should be discussed at a multidisciplinary conference. Tumors 2 cm or larger are at risk for lymph node or distant metastasis and a right hemicolectomy is indicated. Also, for tumors with invasion into the mesoappendix, lymphovascular invasion, or increased mitotic activity (Ki index >3%), a right hemicolectomy should be considered.
Goblet Cell Carcinoids
The most common presentation is appendicitis but could also be a bowel obstruction, intussuception, gastrointestinal bleeding, and chronic lower abdominal pain. More than 50% of patients present with metastatic disease and frequently an appendiceal primary is not considered. This is more common in women, who have ovarian metastasis and are misdiagnosed as having ovarian primary. None of the patients presents with carcinoid syndrome, and urinary 5-hydroxyindoleacetic acid levels and other neuroendocrine markers are usually within normal limits. The clinical outcome of GCC is more favorable than stage-matched adenocarcinoma of the appendix. The most common route of metastasis is transcoelomic but metastasis to lymph nodes, ribs, and vertebra are also reported. Stage and grade of the tumor are important prognostic factors. High mitotic activity, high Ki index greater than 3%, nodal involvement, angioinvasion, and increased mucin production indicate aggressive behavior.
Epithelial Tumors
Patterns of presentation vary widely, which adds to the inability of initial care providers to diagnose correctly the cause of appendiceal neoplasms. The tumors present as an incidental finding in the appendectomy specimen, for appendicitis, as a pelvic mass or as peritoneal carcinomatosis with or without ascites. Appendicitis is a common presentation in men and woman combined but peritoneal dissemination of mucinous appendiceal neoplasm is an important initial presentation. Patients also present to primary care physicians with abdominal distension, increasing abdominal girth, fatigue, weight gain, shortness of breath, and early satiety. Women are usually referred to a gynecologist for possible ovarian cancer. A high percentage of patients are referred to general surgeons after debulking gynecologic surgery for pelvic masses presumed of ovarian origin. Umbilical, inguinal, and incisional hernias filled with mucin, discovered at the time of hernia surgery, is another mode of presentation.
When the mucin extrudes through the appendicular wall due to increased intraluminal pressure, the mucin-producing tumor cells are released into the free peritoneal cavity ( Fig. 1 ). The flow of mucin follows that of peritoneal fluid and circulates in a clockwise direction from the right paracolic sulcus, right subdiaphragmatic area, retrohepatic vena cava, left diaphragm, splenic hilum, and ligament of Treitz. The falciform ligament directs flow to the pelvis, the cul de sac, left paracolic sulcus, and ovaries. The small bowel due to peristalsis is generally initially not involved with the mucinous implants.
Diagnosis
The goals of the work-up include staging of the appendiceal tumor, characterizing the biologic behavior by its histology, clinical history, physical examination, and radiologic studies and deciding if surgery should be a part of the treatment plan. The authors recommend summarizing the final work-up and pretreatment diagnosis by a team familiar with treatment of appendiceal neoplasms and patients with peritoneal carcinomatosis. The reason for this recommendation is that patients could be denied surgical treatment and a chance for long-term survival based on widespread belief that peritoneal carcinomatosis is a contraindication for aggressive surgery. Only an experienced cytoreduction surgeon could determine if complete removal of all visible tumor is possible or if debulking surgery for palliation could help improve the quality of life (QOL) in patients with large accumulation of mucin. As discussed previously, the clinical presentation should be a clue to the physician about a potential appendiceal neoplasm. The physical examination of such patients should also include a digital rectal and pelvic examination to assess for masses in the pelvis and the mobility of these structures to the surrounding anatomy. It is confirmed by preoperative imaging studies, intraoperative findings, or postoperative pathology results. Delay in diagnosis is a common problem due to lack of understanding of the pathophysiology of this condition. The authors’ group presented data that the time from diagnosis to treatment with CRS and HIPEC of more than 6 months correlated with worse outcome.
Preoperative Studies
CT
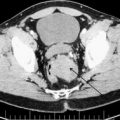
Most patients have a CT scan of the abdomen and pelvis before being referred to a peritoneal surface malignancy program. Findings suggestive of an appendiceal neoplasm are appendiceal dilation or mass and gross ascites with a mucinous component. The mucin maybe seen distributed as discussed previously or in hernia sacs when present. There is nodularity of the lining of the diaphragm and potential indentations on the surface of the liver from the solid component of mucin. The mesentery of the small bowel could be foreshortened from the fibrotic reaction of the tumor in the mesentery leading to a mushroom-shaped image on CT scan ( Fig. 2 ). Rarely, hydroureter ( Fig. 3 ) is seen when tumor involves the vesicoureteral junction, which may have compromised renal function Fig. 4 shows extensive involvement of all areas of the peritoneal cavity. PET scan have not shown promise in the case of DPAM or PMCA. The use of MRI is attractive due to reduced radiation exposure but its use should be determined based on the expertise of a radiologist to read abdominal MRI.
Tumor markers
Carcinoembryonic antigen (CEA), CA19-9, and CA-125 are potential tumor markers in epithelial appendiceal neoplasms. One or any combination of them can be elevated in 60% of patients. A preoperative level is routinely done. Their use is primarily for follow-up and as a response to therapy. After CRS and HIPEC, they are helpful in following a patient’s clinical status and are indicative of recurrence. The elevation can precede the CT findings by several months. None of them is specific to either DPAM or PMCA. Multiple abnormal tumor markers were not useful as an exclusion criterion for patients undergoing CRS. The 3-year survival rates in patients with elevated versus nonelevated CA-125 levels were 83% versus 52% ( P = .003); hence, an elevation in CA-125 is an important indicator of survival in these patients.
Preoperative Evaluation for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
A thorough history and physical examination is important. Laboratory studies, including complete blood cell count and complete metabolic panel with tumor markers, CEA, cancer antigen (CA) 19-9, and CA-125, are obtained. A CT scan of the chest abdomen and pelvis with oral and intravenous (IV) contrast is important in assessment. Preoperative clearance studies in the form of ECG or chest radiograph (if no CT scan) are obtained. Cardiac stress test is indicated when patients are older than 65 years or have a positive cardiac history. The patients are also required to have a colonoscopy to assess for any polyps or masses. Split renal function studies are indicated only in a rare situation where a nephrectomy maybe considered necessary.
Contraindications to CRS/HIPEC
Absolute
- 1.
Extension outside the peritoneal cavity
- 2.
Biliary obstruction
- 3.
Multiple small bowel obstructions
Relative
- 1.
Poor functional status of patient
- 2.
Cardiac contraindication
- 3.
Foreshortened mesentery that would result in postoperative short bowel syndrome. Although the CT scan can suggest it, it is not always a reliable sign.
Parenchymal involvement of the liver, which is rare in appendiceal neoplasms, is not a contraindication to CRS and HIPEC but should be amenable to a complete resection.
Surgical technique and postoperative care
Laparoscopy can be utilised to access the extent of small bowel involvement, but in patients with extensive disease in the omentum and previous surgeries, it is difficult to evaluate completely the extent of small bowel involvement. It is imperative to place all laparoscopic ports in the midline in an effort to reduce port site recurrences that could complicate further surgery. The authors’ group has demonstrated a postsite recurrence of 34% of port sites resected. The extent of small bowel resection determines the QOL after cytoreduction. Every effort should be made to minimize bowel resection even if it requires multiple anastomosis or wedge resections to accomplish that goal.
Under general anesthesia, a midline xiphopubic incision is used to gain access to the abdominal cavity. Tumor burden is calculated using the peritoneal cancer index (PCI), as reported by Sugarbaker ( Fig. 5 ).
Lesion size score is applied to each of the 9 abdominopelvic regions, the jejunum, and the ileum. Summation of the lesion size score gives the PCI (range = 1–39). Surgical resection of the primary tumor is done followed by peritonectomy procedures originally described by Sugarbaker. The extent of surgery is determined by the size and location of the tumor. The objective is to remove all visible tumor (complete cytoreduction). Complete resection is defined as completeness of cytoreduction score, CC-0 or CC-1 ( Box 1 ).
CC-0 = No visible tumor
CC-1 = 0–0.25 cm
CC-2 = 0.25–2.5 cm
CC-3 = >2.5 cm
Findings at surgery could be consistent with appendiceal mass, mucin in the abdomen, bowel obstruction from mucinous component, foreshortened mesentery, peritoneal implants, diaphragmatic implants, masses, or nodules at previous laparoscopic port sites.
Peritonectomy procedures are done as needed to achieve a good cytoreduction and may include anterior abdominal wall peritonectomy, greater omentectomy and splenectomy; left and right upper quadrant peritonectomies with stripping of the respective hemidiaphragms, which requires the placement of chest tubes; lesser omentectomy with cholecystectomy and striping of the omental bursa and porta hepatis; or pelvic peritonectomy with total abdominal hysterectomy and bilateral salpingo-oophorectomy with/without anterior resection of the rectum. Visceral peritonectomy and resection is frequently needed to accomplish this goal. Final assessment of cytoreduction is recorded based on the CC score (see Box 1 ).
After the cytoreduction and before any anastomosis is made, HIPEC is performed intraoperatively with mitomycin C for 90 minutes at a total dose of 40 mg (30 mg given initially and 10 mg added after half an hour of perfusion) using a closed technique. The outflow temperature is maintained at 41°C to 42°C. Urine output is maintained (250–400 mL/h) by using crystalloids and albumin to prevent renal toxicity. During the perfusion the patient is shaken manually and the operating table is positioned in different directions every 15 minutes. On completion of perfusion, the abdomen is opened and gastrointestinal reconstruction is done as appropriate.
Patients are transferred to an ICU where hemodynamic parameters and fluid status are carefully monitored. The authors’ practice is to place chest tubes bilaterally immediately postoperatively when diaphragmatic peritonectomy is performed. The Jackson-Pratt drains that are placed in the Morison pouch, pelvis, and near the tail of pancreas are also monitored. Patients are subsequently transferred to the surgical floor when stable. Usually the following morning, physical therapy is started on postoperative day 1 and early mobilization is encouraged. Deep vein thrombosis prophylaxis is implemented during and after using compression stockings, low-molecular-weight heparin, and early mobilization. Patients are discharged from hospital when clinically stable. Patients from out of town are requested to stay in town to make sure they can maintain good hydration and nutrition. It important to be proactive because patients become dehydrated and consult late, leading to increased rate of readmissions. Baseline clinical assessment with complete physical examination; CT scan or MRI of chest, abdomen, and pelvis; and tumor markers is done at 2 months postoperatively, then every 6 months for the first 5 years, and then yearly until year 10.
Important Technical Considerations
Open or closed HIPEC
The open and closed techniques for intraperitoneal hyperthermic chemotherapy have been used. To date, no conclusive evidence exists that one is superior to another.
Initial entrance into abdominal cavity
Most patients present to the authors with multiple previous surgeries and with advanced disease. Entrance into the abdomen should be performed carefully because frequently adhesions to the small bowel can lead to multiple enterotomies and consequently increased small bowel resections and increased complications. If possible, the abdominal cavity is entered high in the epigastric region where the liver or stomach can be found easily with less incidence of bowel adhesions.
Approach to the porta hepaticus during CRS
CRS and HIPEC have become important options for patients with peritoneal carcinomatosis. The CC determines survival. Frequently, the porta hepaticus and the lesser sac are massively involved by tumor ( Fig. 6 ). Encasement of portal triad, lesser omentum, retrohepatic vena cava, duodenum, and stomach is frequently seen. The proximity to major portal structures and retrohepatic vena cava makes this dissection challenging. In the authors’ experience, this is the area where meticulous surgical technique and expertise are necessary to obtain complete removal of all tumor. Some specific technical considerations are important to assure that all tumor is safely removed. These are
- •
Determining the extent of resection of other areas of the abdomen to assure the level of cytoreduction that can be accomplished
- •
Determining the extent of bowel resection needed to evaluate if a partial gastrectomy will significantly worsen QOL
- •
Performing right and left diaphragmatic peritonectomies and mobilizing all ligaments of the liver to obtain adequate liver mobility with the dissection of the round ligament at the end
- •
Completing mobilization of the greater curvature of stomach to evaluate the extent of involvement of the lesser sac
- •
Performing cholecystectomy and peritonectomy over the infrahepatic vena cava to gain access to the foramen of Winslow and portal triad. Peritonectomy at this level needs to be extended as far as possible to the retrohepatic vena cava and segment 1 of the liver, including the posterior aspect of the portal triad. This maneuver makes the dissection from the lesser sac easier.
- •
If the portal triad is not accessible anteriorly, as is frequently the case, it should be approached through the gastrocolic space (see Fig. 6 ). Performing the peritonectomy over the pancreas, where there is frequently large tumor bulk, facilitates the exposure to the celiac trunk and hepatic artery. Once the hepatic artery is identified and protected, the dissection can be carried anteriorly to the portal trial ( Fig. 7 ) by transecting the tumor and separating stomach and duodenum from the porta hepaticus (see Fig. 6 ). The dissection is continued along the anterior aspect of the portal triad toward the base of the round ligament.