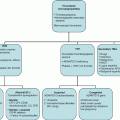
Chapter 31 Michael Richards Department of Paediatric Haematology, Leeds Children’s Hospital, LeedsUK Red cell production in the foetus occurs initially in the liver but shifts to the bone marrow during the third trimester of gestation. It is responsive to the effect of the glycoprotein hormone erythropoietin, production of which moves from the liver to the kidney near term. Erythropoietin concentrations fall following delivery, remaining depressed until 6–8 weeks of age; the resulting decreased rate of erythropoiesis results in a reduction in haemoglobin concentrations with a nadir around 10–12 weeks that rarely falls below 100 g/L [1]. The normal range of haemoglobin concentrations during the neonatal period varies according to the gestational age of the infant at birth and the postnatal age of the infant. Increasing erythropoietin levels lead to a rise in the haemoglobin concentration until 6 months of age. This dynamic process makes it essential to refer to the appropriate age-adjusted normal range for haemoglobin concentration in the interpretation of test results during the first 6 months of life [2]. The peak haemoglobin concentration occurs during the first day following delivery, this level being higher than that seen in cord blood samples. The haemoglobin concentration subsequently declines as does the mean red corpuscular volume (MCV). The percentage reticulocyte count decreases from 3–7% on day 1 to 0–1% on day 7 [2]. Examination of the blood film is notable by the presence of nucleated red cells on day 1, which have largely disappeared by the third day of life and are absent in normal older infants. Blood samples obtained by a skin prick represent capillary blood and have a higher haemoglobin concentration than blood obtained by venous sampling. In the first hours following delivery, this difference averages approximately 35 g/L, but in some patients, this difference may exceed 100 g/L [3]. This difference is most notable in premature infants and those who are clinically unstable displaying acidosis or hypertension. The impact of the sampling technique becomes less significant as infants approach full-term gestation and by the end of the first week of life. The blood volume and red cell mass of the infant are influenced by the treatment of the umbilical cord blood vessels. Shortly after birth, the mean blood volume of term infants has been determined at 85 mL/kg, ranging from 50 to 100 mL/kg. Placental blood vessels contain 75–125 mL of blood at birth, which represent 25–75% of the foetal blood volume. The umbilical arteries generally constrict after delivery; however, the umbilical veins remain dilated; newly delivered infants held below the level of the placenta therefore gain blood from the umbilical vein; however, those who are held above the placenta may bleed into it. Early clamping of the umbilical cord will result in a reduction in the volume of blood transfused from the placenta to the neonatal circulation. Usher et al. reported that infants for whom the clamping of the cord was delayed had an average blood volume of 93 mL/kg at an age of 72 h compared to 82 mL/kg in those following immediate cord clamping [4]. There is some evidence suggesting that reduced blood volume is associated with increased mortality in premature infants; conversely, delayed clamping may lead to circulatory overload and congestive cardiac failure. Premature infants (<37 weeks of gestation) experience an earlier (4–6 weeks) and lower nadir concentration of haemoglobin (70 g/L if birth weight <1000 g) than full-term babies, resulting in a normochromic, normocytic anaemia with a low reticulocyte count that is mostly due to impaired erythropoietin production and phlebotomy blood loss [1]. Some premature infants tolerate this anaemia well, but others especially the very low-birth-weight and sick infants demonstrate adverse signs such as tachycardia, tachypnoea, apnoea, poor weight gain, increased oxygen requirement, diminished activity and pallor. Management options for these infants have included red cell transfusion and the use of exogenous recombinant erythropoietin. There are no data to suggest that recombinant erythropoietin dramatically decreases or eliminates the need for red cell transfusions in preterm infants although there is a suggestion that its use may prevent red cell transfusion in extremely low-birth-weight infants (birth weight <1000 g) after 1 month of age [5]. There are limited, non-corroborated data that suggest that retinopathy of prematurity (a disorder of vascular proliferation) may be exacerbated by recombinant erythropoietin. Red cell transfusion remains the mainstay of treatment for anaemia of prematurity; recent developments have concentrated on the need to restrict the use of transfusions to cases which satisfy strict criteria. Specific haemoglobin targets vary according to postnatal age and cardiorespiratory status. Ensure the infant is iron replete, especially if breastfed. Strategies to reduce red cell loss include delayed clamping of the umbilical cord and reducing the frequency and volume of blood sampling. As in older patients, anaemia in the neonate may be categorized into pathology leading to bleeding or haemolysis, and disorders that compromise production of red cells from the bone marrow. The causes of anaemia are summarized in Table 31.1, and an approach to the investigation of anaemia in neonatal intensive care is outlined in Figure 31.1. The role of transfusion in neonatal intensive care is discussed in Chapter 34. Table 31.1 Causes of neonatal anaemia.
Neonatal Anaemia
Normal development of red cell production during the foetal and neonatal period
Normal blood values and impact of technical issues
Anaemia of prematurity
Anaemia in the neonate
Inadequate production
Foetal/neonatal blood loss
Increased red cell consumption
Exaggeration of physiological anaemia as seen in premature infants
Twin-to-twin transfusion, foetal to maternal haemorrhage, placenta praevia
Immune-mediated haemolysis secondary to Rhesus disease of the newborn and other red cell antigen incompatibilities, maternal autoimmune haemolytic anaemia
Bone marrow failure syndromes, e.g. Diamond–Blackfan anaemia, Fanconi anaemia, Down’s syndrome
Early clamping of the umbilical cord
Microangiopathic haemolytic anaemia secondary to arteriovenous malformation
Haematinic deficiency iron deficiency, rarely B12 and folate deficiency
Significant internal haemorrhage including intracranial haemorrhage and cephalohaematoma
Red cell membrane disorders
Disorders of haemoglobin synthesis including severe alpha thalassaemia
Venous sampling for investigations
Red cell enzyme deficiency
Infiltration of the bone marrow by leukaemia, lymphoma, neuroblastoma, osteopetrosis
Vitamin E deficiency
Bone marrow suppression by viruses, notably parvovirus
Parasitaemia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree