Lessons have recently been learned in the use of neoadjuvant chemotherapy. This article explains how diagnosis of a pathologic complete response (pCR) can avoid an unfavorable prognosis in patients with high-risk breast cancer; how the surrogacy of pCR for long-term survival remains questionable; how translational biomarker studies have not been helpful in identifying patients with a high chance of treatment benefit; assessment of the prognosis of patients without a pCR for identifying patients at high risk and which clinical trials will be available for these patients in the near future; and which patients might require less locoregional treatment.
Key points
- •
Recent lessons have been learned that will increase the use of neoadjuvant chemotherapy.
- •
With the evolving knowledge on how best to perform neoadjuvant chemotherapy in the various cancer subtypes and how to use the information gained for the individual patients, a trend toward more frequent use of this approach can be observed.
- •
This article provides important prognostic information for those patients with unfavorable initial prognosis for achieving a pathologic complete response and allows subsequent treatment to be adopted accordingly.
- •
The response-guided approach might be an option for further improving treatment, especially in hormone receptor–positive tumors.
- •
Neoadjuvant treatment will become standard of care when health care authorities approve the first innovative agent based on a neoadjuvant or postneoadjuvant study and patients will have to be treated neoadjuvantly to get access to the new treatment options.
Introduction
The concept of moving systemic treatment from after surgery to before surgery was first developed based on a hypothesis that the earlier disseminated single tumor cells are killed the less likely is the development of metastasis at a later stage. However, trials conducted in the 1980s and 1990s have not confirmed this concept but have found similar outcomes in patients treated with chemotherapy before or after surgery. Thereafter neoadjuvant chemotherapy was mainly used to obtain a better operability of large operable or even inoperable locally advanced tumors. This approach is now considered as a model to learn not only about the biology of breast cancer in general but also about the treatment sensitivity of an individual patient’s tumor ( Fig. 1 ). Tumor tissue obtained by core biopsy before treatment allows biological characterization of the tumor and development of an individual treatment plan. Early assessment after the first couple of cycles allows testing for functional predictors but also the changing of treatment according to early response. After completing all neoadjuvant treatment, histologic examination of the surgically removed breast and axillary tissue allows precise assessment of tumor response and characterization of residual, treatment-resistant disease.
These arguments for neoadjuvant therapy have led to its broader use in smaller and operable disease; however, adjuvant treatment remained the first option of care in most parts of the world. This article discusses several lessons learned recently that might support the use of neoadjuvant chemotherapy as standard of care in some, mainly more aggressive, subtypes of breast cancer.
Introduction
The concept of moving systemic treatment from after surgery to before surgery was first developed based on a hypothesis that the earlier disseminated single tumor cells are killed the less likely is the development of metastasis at a later stage. However, trials conducted in the 1980s and 1990s have not confirmed this concept but have found similar outcomes in patients treated with chemotherapy before or after surgery. Thereafter neoadjuvant chemotherapy was mainly used to obtain a better operability of large operable or even inoperable locally advanced tumors. This approach is now considered as a model to learn not only about the biology of breast cancer in general but also about the treatment sensitivity of an individual patient’s tumor ( Fig. 1 ). Tumor tissue obtained by core biopsy before treatment allows biological characterization of the tumor and development of an individual treatment plan. Early assessment after the first couple of cycles allows testing for functional predictors but also the changing of treatment according to early response. After completing all neoadjuvant treatment, histologic examination of the surgically removed breast and axillary tissue allows precise assessment of tumor response and characterization of residual, treatment-resistant disease.
These arguments for neoadjuvant therapy have led to its broader use in smaller and operable disease; however, adjuvant treatment remained the first option of care in most parts of the world. This article discusses several lessons learned recently that might support the use of neoadjuvant chemotherapy as standard of care in some, mainly more aggressive, subtypes of breast cancer.
Lesson 1: diagnosis of a pathologic complete response avoid unfavorable prognosis in patients with high-risk breast cancer subtypes
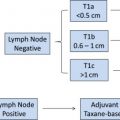
Recent results from a meta-analysis of 7 German neoadjuvant studies revealed that pathologic complete response (pCR) is not prognostic for disease-free and overall survival for all subtypes of breast cancer. Subtypes were defined using estrogen and progesterone receptor status, human epidermal growth factor receptor 2 (HER2) receptor status, and tumor grade according to recent St Gallen recommendations. Patients with luminal B (HER2-negative)–like (defined as hormone receptor [HR]–positive, grade 3 tumors), HER2-positive (nonluminal)–like, and triple-negative breast cancers that achieved a pCR showed a better outcome compared with those without a pCR.
However, in patients with luminal A–like (defined as HR-positive, grade 1 or 2 tumors) or luminal B (HER2-positive)–like (defined as HR-positive, HER2-positive, any grade) breast cancers, prognosis was not significantly different for patients with and without a pCR. It therefore seems that pCR as a surrogate marker for survival for patients with these two subtypes is less reliable ( Fig. 2 ).
Comparing patients with pCR of the various subtypes, it could be shown that the subtypes in which pCR showed a prognostic impact had better outcomes than the other two luminal subtypes, despite initially being considered as more aggressive. Based on these observations, patients with these three subtypes having achieved a pCR can be informed that their prognosis has turned from poor to highly favorable (see Fig. 2 ).
The Food and Drug Administration (FDA) of the United States recently gathered data not only from these German studies but also from other large-scale trials around the world. A total of 12,963 patients were collected, including 2 trials from National Surgical Adjuvant Bowl and Breast Project (NSABP), 2 from European Cooperative Trials in Operable breast cancer (ECTO), and 1 from European Organization for Research and Treatment of Cancer (EORTC)/Breast International Group (BIG) groups. A similar analysis to that described earlier was conducted confirming the results for the more aggressive tumor types; however, marginal differences in favor of patients with a pCR were also observed for patients with luminal A–like ( P = .07) and luminal B (HER2-positive)like ( P = .023 for patients having not received trastuzumab and P = .028 for patients having received trastuzumab). Hazard ratios (HRs) for event-free survival between patients with and without a pCR were in the range of 0.58 and 0.63 for luminal A–like and luminal B (HER2-positive)like tumors and in the range of 0.24 to 0.27 for triple-negative, luminal B (HER2-negative)like, and HER2-positive (nonluminal)like tumors. The less extensive HRs in the first two subgroups again reflect the less favorable outcome of patients with a pCR. Outcomes of patients with a pCR in HER2-positive (nonluminal)like tumors improved substantially when trastuzumab was part of the neoadjuvant treatment.
Lesson 2: pCR should request no invasive (and no noninvasive) residuals in the breast and nodes; however, surrogacy with long-term survival is questionable
Two questions have to be answered: the first is regarding the optimal definition, and the second is regarding whether a change in pCR rate by, for example, a better treatment is directly associated with a change in survival time in the same direction.
Various definitions of pCR have been proposed by different groups. When systemic treatment was less effective, patients with even minimal residual invasive disease were considered as a pCR (ypT<1a ypN±); others have considered only response of the breast tumor and considered all patients without invasive tumor residuals in the breast irrespective of nodal status as a pCR (ypT0/is ypN±). With more effective treatments becoming available, definitions became more conservative, requesting either no invasive residuals in breast and nodes (ypT0/is ypN0) or even no invasive and no noninvasive residuals in all removed tissues from breast and axillary nodes (ypT0 ypN0). The comparison of these definitions was first addressed by the meta-analysis of the German studies showing that the HR between patients with and without pCR was highest for the most conservative definition ypT0 ypN0 (HR 4.04 for disease-free survival [DFS] and 7.39 for overall survival [OS]) and decreased with less conservative definitions (3.51 for DFS and 5.99 for OS for ypT0/is ypN0; 2.77 for DFS and 3.66 for OS for ypT0/is ypN±; 2.11 for DFS and 2.80 for OS for ypT<1a ypN±). Again the FDA addressed the same question in their global meta-analysis and found that around 5% of patients have only in situ residuals (ypTis ypN0) and another 4% of patients have no invasive residuals in the breast but involved lymph nodes (ypT0/is ypN+), whereas 13% of patients achieved the most complete pCR (ypT0 ypN0). When plotting Kaplan-Meyer curves of patients with ypT0 ypN0, ypT0/is ypN0 and ypT0/is ypN± disease in the same diagram, no relevant difference in event-free survival and OS was observed between the first 2 curves, but the last 1 showed an inferior outcome. The conclusion therefore is that both ypT0 ypN0 as well as ypT0/is ypN0 are appropriate definitions for pCR. Because the rate of residual disease varies between tumor subtypes (highest in HER2-positive disease) the choice of the definition in clinical trials probably depends on the assumptions to be made for sample size calculation. For routine care, both definitions are suitable.
Because the FDA is currently considering accepting neoadjuvant studies as a new pathway for early drug registration, an important aim of their meta-analysis concerned the second question: what magnitude of pCR improvement in a randomized trial predicts long-term clinical benefit? To answer this question, odds ratios on pCR of the randomized treatment arms were plotted against HRs of event-free survival. A straight diagonal line ideally shows that small increases in pCR rate are correlated with small improvements in event-free survival, and large increases in pCR rate are correlated with large improvements in event-free survival. However, the analysis found a horizontal line, meaning that such a correlation does not exist.
However, there are some suggestions in the literature that this might be the case, at least for patients with HER2-positive disease.
- •
In the NOAH (NEoAdjuvant Herceptin) study, comparing patients receiving neoadjuvant chemotherapy with or without trastuzumab, it was shown that the pCR rate could be increased by 20% with the combined treatment. In addition, a significant improvement in event-free survival was reported ( Fig. 3 ). This trial therefore led to the approval of trastuzumab for neoadjuvant treatment by the European Medical Agency.
Fig. 3
Increase in pCR rates by adding trastuzumab to anthracycline-taxane–based chemotherapy correlates with improved outcome in the NOAH study. ∗For comparison of HER2-positive disease groups.
( Adapted from Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010;375(9712):377–84; with permission.)
- •
Several trials have compared the tyrosine-kinase inhibitor lapatinib with trastuzumab. In all trials there was a trend toward a lower pCR rate for the combination of chemotherapy with lapatinib. The largest of these trials, the GeparQuinto study, even showed a significantly inferior pCR rate for patients receiving 24 weeks of lapatinib and anthracycline-taxane–containing chemotherapy. These results are consistent with the closure of one arm of the ALTTO (adjuvant lapatinib and/or trastuzumab treatment optimazation) trial, which used lapatinib as the only adjuvant anti-HER2 treatment based on the results of an early interim efficacy analysis ( Fig. 4 ).
Fig. 4
Results of the GeparQuinto trials showing inferior pCR rates with lapatinib compared with trastuzumab in combination with neoadjuvant chemotherapy correspond with an early closure of the lapatinib-alone arm of the adjuvant ALTTO study.
( Data from Untch M, Loibl S, Bischoff J, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol 2012;13(2):138.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree