Fig. 11.1
Preoperative vs. postoperative radiotherapy. Survival rate in the postoperative radiotherapy-alone group (B) was significantly better than that in the pre- plus postoperative radiotherapy group (A)
11.2.1.2 Postoperative Chemotherapy
Postoperative Radiotherapy vs. Postoperative Chemotherapy
Cisplatin has been available as a key drug in the treatment of esophageal cancer in Japan since the early 1980s. The third JEOG RCT was performed to determine which postoperative therapy provided better survival: radiotherapy or chemotherapy. This study (JCOG8503, 1984–1987) compared postoperative radiotherapy (50 Gy) with postoperative chemotherapy (70 mg/m2 cisplatin plus 3 mg/m2 vindesine × 2 courses). The chemotherapy regimen of cisplatin plus vindesine was adopted in this study because this combination was the standard regimen for non-small cell lung cancer at that time, when cisplatin plus 5-FU was not yet popular. Although this study showed no significant difference in the 5-year overall survival rate between the two groups [11] (Fig. 11.2), the results did suggest, however, that postoperative chemotherapy including cisplatin was not inferior to postoperative radiotherapy, the standard treatment modality at that time. As a result, postoperative chemotherapy gained common acceptance as adjuvant therapy for ESCC in Japan.
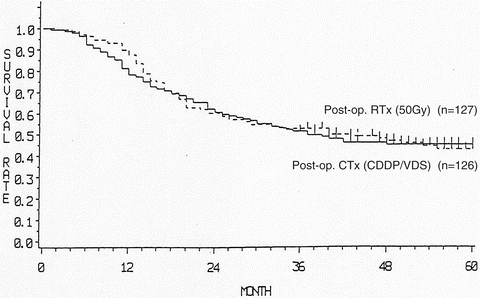

Fig. 11.2
Postoperative radiotherapy vs. postoperative chemotherapy. The 5-year survival rate was 44 % in the postoperative radiotherapy group and 42 % in the postoperative chemotherapy group, showing no significant difference between the two groups
Additive Effect on Survival of Postoperative Adjuvant Chemotherapy over Surgery Alone
Esophageal cancer surgery showed improved quality of lymphadenectomy, including specific dissection of the cervico-upper mediastinal nodes, which became the standard practice in the late 1980s in Japan. Therefore, in the fourth JEOG RCT, it was considered necessary to determine whether postoperative adjuvant chemotherapy conferred a survival benefit on patients undergoing radical esophageal cancer surgery. This study (JCOG8806, 1988–1991) compared surgery alone with surgery plus postoperative chemotherapy (70 mg/m2 cisplatin plus 3 mg/m2 vindesine × 2 courses). This study showed no significant difference in the 5-year overall survival (OS) rate between the two groups [12] (Fig. 11.3). Based on this result, surgery alone became the standard of care for ESCC at that time.
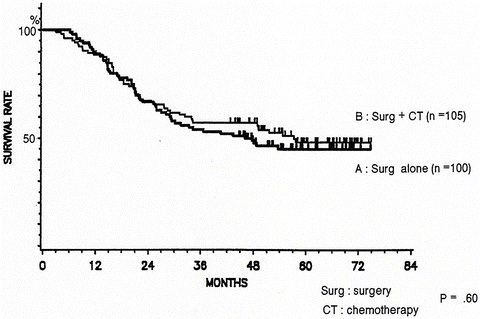

Fig. 11.3
Surgery alone vs. postoperative chemotherapy (cisplatin + vindesine). The 5-year survival rate was 45 % in the surgery-alone group and 48 % in the postoperative chemotherapy group, showing no significant difference between the two groups
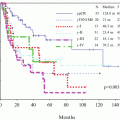
The efficacy of combination of cisplatin and 5-fluorouracil (5-FU) in patients with advanced esophageal cancer was superior to that of cisplatin and vindesine, based on our experience of two phase II studies. The fifth JEOG RCT was, therefore, initiated to determine whether postoperative adjuvant chemotherapy using cisplatin and 5-FU had an additive effect on survival in patients undergoing radical surgery alone for pathologic stage II or III, excluding T4, squamous cell carcinoma. This study (JCOG9204, 1992–1997) compared surgery alone with surgery plus postoperative chemotherapy (80 mg/m2 cisplatin on day 1 plus 800 mg/m2 5-FU on days 1–5 × 2 courses). The 5-year disease-free survival rates (primary endpoint) were 45 % in the surgery-alone group (122 patients) and 55 % in the postoperative chemotherapy group (120 patients) (p = 0.04), while the 5-year overall survival rates (OS) were 52 and 61 %, respectively, (p = 0.13). Risk reduction by postoperative chemotherapy was remarkable in the subgroup with lymph node metastasis [13] (Fig. 11.4a, b). On the basis of these data, postoperative adjuvant chemotherapy using cisplatin and 5-FU came to be considered the standard of care for patients with ESCC in the early 2000s.
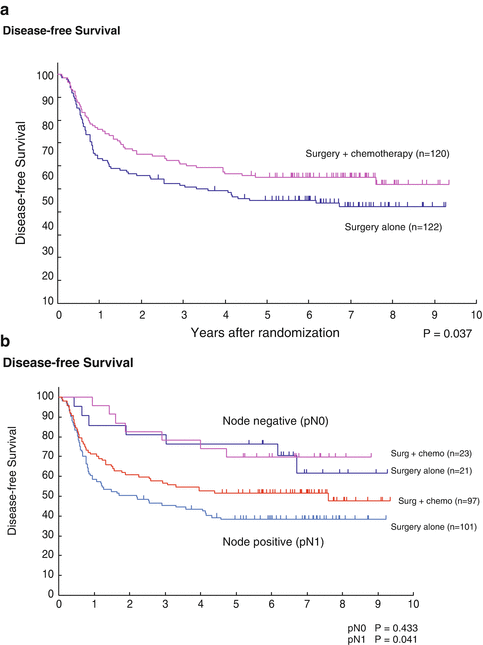

Fig. 11.4
(a) Surgery alone vs. postoperative chemotherapy (cisplatin + 5-FU). Disease-free survival curves of all registered patients. The 5-year disease-free survival was 45 % in patients with surgery alone and 55 % in patients with surgery plus chemotherapy (p = 0.037). (b) Surgery alone versus postoperative chemotherapy (pN0/pN1). In the pN0 subgroup, the 5-year disease-free survival was 76 % in the surgery-alone group and 70 % in the surgery plus chemotherapy group (p = 0.433); in the pN1 subgroup, it was 38 % in the surgery-alone group and 52 % in the surgery plus chemotherapy group (p = 0.041)
11.2.1.3 Preoperative Chemotherapy (Neoadjuvant Chemotherapy)
Even though postoperative adjuvant chemotherapy was considered the standard of care for esophageal cancer patients in Japan, preoperative treatment still predominated in Western countries due to the invasiveness of esophageal cancer surgery and the attending high morbidity [14]. Therefore, the positive role of preoperative chemotherapy regarding survival in patients with esophageal cancer compared with surgery alone or postoperative chemotherapy remained controversial. Details regarding this controversy are described in the next subchapter. The sixth JEOG RCT was, therefore, initiated to determine the optimal perioperative timing of chemotherapy in patients with locally advanced ESCC, that is, before or after surgery. In this study (JCOG9907, 2000–2006), eligible patients with clinical stage II or III, excluding T4, SCC were randomly assigned to undergo surgery either followed (Post group) or preceded (Pre group) by chemotherapy (80 mg/m2 cisplatin on day 1 plus 800 mg/m2 5-FU, continuous infusion (c.i.) over days 1–5 × 2 courses with a 3-week interval). Progression-free survival, the primary endpoint, did not reach the discontinuation boundary, but OS in the Pre group (164 patients) was superior to that in the Post group (166 patients) (p = 0.01). Updated analyses showed that the 5-year OS was 43 % in the Post group and 55 % in the Pre group (hazard ratio, 0.73; 95 % confidence interval, 0.54–0.99; p = 0.04) [15] (Fig. 11.5a, b). Though renal dysfunction after surgery in the Pre group was slightly higher than that in the Post group, preoperative chemotherapy did not increase the risk of complications or hospital mortality after surgery [16]. There are three possible reasons for the better preoperative chemotherapy results. First, downstaging was achieved in some patients by preoperative chemotherapy. While the proportion of the patients with clinical stage II disease was similar in the two groups, the proportion with pathologic stage II or lower was greater in the Pre group. Second, complete resection (R0) was slightly more frequent in the Pre group than the Post group. Third, the rate of completion of the protocol treatment was much better in the Pre group than the Post group. Treatment according to the protocol with two courses of chemotherapy and R0 resection was done in 85.4 % of the Pre group patients but only in 75.0 % of patients in the Post group.
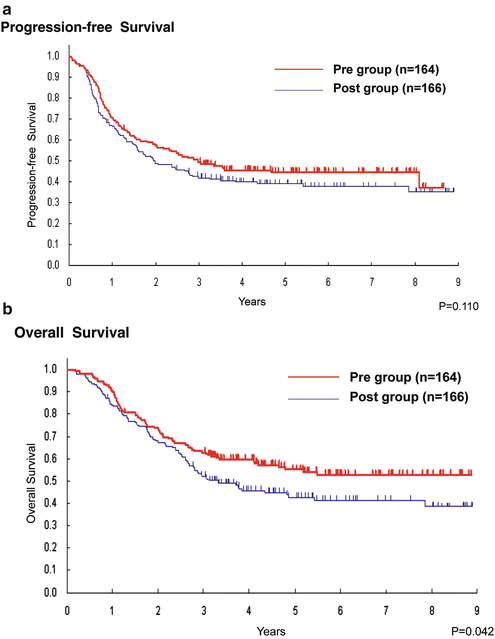

Fig. 11.5
(a) Preoperative vs. postoperative chemotherapy. Progression-free survival. Pre group = preoperative chemotherapy, Post group = postoperative chemotherapy. No significant difference was observed in progression-free survival between the two groups. (b) Preoperative vs. postoperative chemotherapy. Overall survival. Pre group = preoperative chemotherapy, Post group = postoperative chemotherapy. The 5-year OS was 43 % in the Post group and 55 % in the Pre group (p = 0.04)
Based on these results, preoperative chemotherapy with cisplatin plus 5-FU came to be regarded as the standard of care for patients with stage II/III SCC, and this treatment modality was described as the new standard of care in the latest revision of the Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus. Thus, the optimal perioperative timing of surgical adjuvant therapy once again became before surgery.
11.2.2 Future Candidates for Surgical Adjuvant Therapy for ESCC in Japan
The results of subgroup analyses in JCOG9907 showed that preoperative chemotherapy was more effective in clinical stage II or T1-2 cases than in stage III or T3, namely in relatively early stage patients. Furthermore, the lower rate of isolated loco-regional recurrence of 31 % among tumor recurrence cases in the postoperative chemotherapy group and of 25 % in the preoperative chemotherapy group may result from our meticulous surgical procedure. The results of our study suggest that preoperative chemotherapy using cisplatin and 5-FU is a good treatment strategy, if sufficient local tumor control is achieved by aggressive surgical procedures, while if local tumor control is insufficient, more aggressive adjuvant therapy such as preoperative chemoradiotherapy with an aim of local tumor control or more intensive preoperative chemotherapy with an aim of systemic disease control may be a preferable treatment modality. Docetaxel is one of the most promising drugs for esophageal cancer and the recently reported exploratory trial of preoperative chemotherapy with docetaxel plus CF (DCF) for locally advanced ESCC showed a good response rate (61.5 %) with no treatment-related deaths [17]. The clinical question of which is better, preoperative chemotherapy or preoperative chemoradiotherapy, still needs to be clarified.
Based on these background features, the JEOG has launched a three-arm randomized controlled trial JCOG1109 to confirm the superiority of DCF and the superiority of chemoradiotherapy with CF (CF-RT) in overall survival over CF as preoperative therapy for locally advanced ESCC [18]. Patients in arm A receive two courses of preoperative CF (80 mg/m2 cisplatin on day 1 plus 800 mg/m2 5-FU, c.i. on days 1–5) repeated every 3 weeks. Patients in arm B receive three courses of preoperative DCF (70 mg/m2 docetaxel on day 1 plus 70 mg/m2 cisplatin on day 1 plus 750 mg/m2 5-FU, c.i. on days 1–5) repeated every 3 weeks. Patients in arm C receive preoperative chemoradiotherapy (41.4 Gy/23 fractions) with two courses of CF (75 mg/m2 cisplatin on day 1 plus 5-FU 1,000 mg/m2 5-FU, c.i. on days 1–4) repeated every 4 weeks (Fig. 11.6).
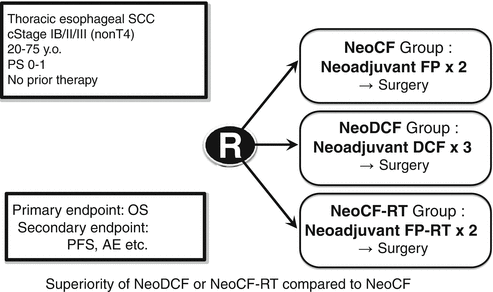

Fig. 11.6
Three-arm phase III trial comparing cisplatin plus 5-FU (CF) vs. docetaxel, cisplatin plus 5-FU (DCF) vs. radiation therapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT Study)
11.3 Adjuvant and Neoadjuvant Therapy for ESCC Out of Japan
Table 11.1 presents a comprehensive overview of the literature-based evidence on adjuvant and neoadjuvant therapies for ESCC out of Japan and from Japan.
Table 11.1
Literature-based evidence on adjuvant and neoadjuvant therapies for ESCC
First author | Accrual period | Stages enrolled | Chemotherapy | Radiotherapy | Surgery | No. of patients | Survival | p value | ||
|---|---|---|---|---|---|---|---|---|---|---|
+ CT, CRT | Surg. alone | + CT, CRT | Surg. alone | |||||||
Adjuvant CT vs. surgery alone | ||||||||||
Pouliquen X [19] (FASR) | 1987–1992 | Excluding T4, N0 | Cisplatin (100 mg/m) | TTE | 68 | 52 | MST: 12 months | MST: 12 months | NS | |
5-FU (1,000 mg/m2) × 6–8 cycles | ||||||||||
Ando [13] (JCOG) | 1992–1997 | IIA, IIB, III, IVa | Cisplatin (80 mg/m2) | TTE | 120 | 122 | 5-year DFS: 55 % | 5-year DFS: 45 % | 0.037 | |
5-FU (800 mg/m2) × 2 cycles | ||||||||||
Lee [20] | 1989–1995 | IIb, III, IVa | Cisplatin (60 mg/m2) | TTE | 40 | 52 (historical control) | 3-year DFS: 47.6 % | 3-year DFS: 35.6 % | 0.049 | |
5-FU (1,000 mg/m2) × 3 cycles | ||||||||||
Neoadjuvant CT vs. surgery alone | ||||||||||
Law [21] | 1989–1995 | Excluding T4 and stage IV | Cisplatin (100 mg/m) | TTE | 74 | 73 | MST: 16.8 months | MST: 13 months | 0.17 | |
5-FU (500 mg/m2) × 2cycles | ||||||||||
Ancona [22] | 1992–1997 | IIA, IIB, III | Cisplatin (100 mg/m) | TTE | 48 | 48 | 5-year OS: 34 % | 5-year OS: 22 % | 0.55 | |
5-FU (1,000 mg/m2) × 2cycles | ||||||||||
1990–1995 | I, II, III | Cisplatin (100 mg/m) | TTE and THE | 213 (54 %; ADC) | 227 (53 %; ADC) | MST: 14.9 months | MST: 16.1 months | 0.53 SCC/ADC NC/NC | ||
5-FU (1,000 mg/m2) × 3cycles | ||||||||||
1992–1998 | Resectable tumor | Cisplatin (80 mg/m2) | TTE and THE | 400 (66 %; ADC) | 402 (67 %; ADC) | MST: 16.8 months | MST: 13.3 months | 0.004 HR: SCC/ADC 0.78/0.78 | ||
5-FU (1,000 mg/m2) × 2 cycles | ||||||||||
Neoadjuvant CT vs. adjuvant CT | ||||||||||
Ando [15] (JCOG) | 2000–2006 | IIA, IIB, III | Cisplatin (80 mg/m2)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||||