Fig. 14.1
The chemical structures of nelarabine and ara-G. (a) Nelarabine. (b) Ara-G
14.2 Mechanisms of Action
14.2.1 Mechanisms of Action (Fig. 14.2)
In the blood, nelarabine is rapidly demethoxylated to ara-G by adenosine deaminase. The ara-G is subsequently transported into cancer cells via p-nitrobenzylthioinosine-sensitive and p-nitrobenzylthioinosine-insensitive transporters, including human equilibrative nucleoside transporters [14]. Ara-G is phosphorylated by either deoxycytidine kinase to cytosolic ara-G monophosphate or by deoxyguanosine kinase to mitochondrial ara-G monophosphate [4]. These nucleotides are further phosphorylated to ara-G triphosphate, an intracellular active metabolite, by nucleotide kinases. Nucleosides are degraded by adenosine deaminase, nucleotidase, or PNP [4]. Ara-G triphosphate competes with intrinsic deoxyribonucleotides as a substrate for incorporation into DNA by DNA polymerases. DNA-incorporated ara-G monophosphate inhibits DNA synthesis and consequently induces apoptosis [13].
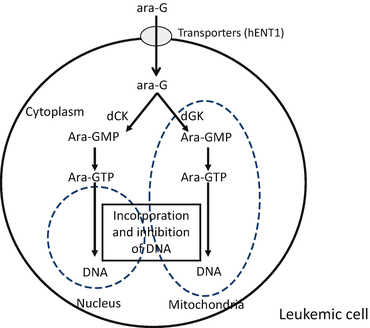

Fig. 14.2
The intracellular activation and the mechanism of action of ara-G. dCK deoxycytidine kinase, dGK deoxyguanosine kinase, ara-GMP ara-G monophosphate, ara-GTP ara-G triphosphate
14.2.2 Mechanisms of Drug Resistance
We developed a novel ara-G-resistant variant of the human T-lymphoblastic leukemia cell line CCRF-CEM, and we investigated the mechanisms behind its resistance to ara-G [15]. The CEM-resistant variant cells were 70-fold more ara-G resistant than the control CEM cells. The transcription level of human equilibrative nucleoside transporter 1 and the protein levels of deoxycytidine kinase and deoxyguanosine kinase were decreased in the resistant cells when compared to the control CEM cells. The production of intracellular ara-G triphosphate was one-fourth that of the control CEM cells. The level of antiapoptotic Bcl-xL was increased, and those of proapoptotic Bax and Bad were decreased in the resistant cells when compared to the control CEM cells. It was concluded that the reduction of drug incorporation into nuclear DNA and the inhibition of apoptosis were the crucial mechanisms behind the ara-G resistance in this cell line.
14.2.3 Adult T-Cell Leukemia/Lymphoma
Adult T-cell leukemia/lymphoma is an aggressive malignancy of mature activated CD4+ T cells that is associated with human T-cell leukemia virus 1 infection. Adult T-cell leukemia/lymphoma is difficult to treat, and the therapeutic outcomes of chemotherapy and allogeneic stem cell transplantation are still unsatisfactory [16]. The cytotoxicity of ara-G was evaluated using 12 cultured adult T-cell leukemia/lymphoma cell lines (ATL-43T, KOB, SO4, M8166, MT-2, MT-4, ED-40515 (+), ED-40515 (−), ST-1-dependent, ST-1-independent, KK1-dependent, and KK1-independent) in vitro [17]. The production of intracellular ara-G triphosphate in these cell lines was less than one-fourth of that in the CEM cell line. Moreover, the majority of the 50% inhibitory concentration values of these cell lines was higher than 1 mM. Thus, ara-G was not cytotoxic to adult T-cell leukemia/lymphoma cells.
14.3 Pharmacokinetics
The pharmacokinetics of nelarabine has been evaluated in patients with hematologic malignancies.
Gandhi et al. conducted a phase I multicenter trial of nelarabine in 26 patients with hematologic malignancies [6]. In their study, nelarabine (20–60 mg/kg) was administered for 5 days. The serum level of nelarabine increased over the duration of the intravenous (IV) infusion, reaching a peak (190 μmol/L) at the end of the infusion (dose of 30 mg/kg); the half-life of nelarabine was 16 min. The peak concentration of ara-G was 115 μmol/L, and it had a half-life of 3.4 h. The median peak intracellular ara-G triphosphate concentrations after the administration of 20, 30, 40, and 60 mg/kg of nelarabine were 23, 42, 85, and 93 pmol/L, respectively. Blasts from patients with T-cell acute lymphoblastic leukemia or T-cell lymphoid blast crisis of chronic myeloid leukemia accumulated relatively greater levels of ara-G triphosphate when compared to other lineages.
Kisor et al. evaluated the pharmacokinetics of nelarabine and ara-G in pediatric and adult patients with refractory hematologic malignancies [18]. Seventy-one patients with leukemia (acute lymphoblastic leukemia, chronic lymphocytic leukemia, acute myeloid leukemia, or chronic myeloid leukemia) or lymphoma (non-Hodgkin lymphoma) were evaluated in this study. The patients received 0.75–2 h IV infusions of nelarabine at 5–75 mg/kg for 5 consecutive days. The maximum concentration of nelarabine occurred at the end of the drug infusion. The harmonic mean half-lives of nelarabine were 14.1 and 16.5 min in the pediatric patients and adult patients, respectively. The maximum concentrations of ara-G, ranging between 11.6 and 308.7 μmol/L, occurred at or near the end of the nelarabine infusion.
Gandhi et al. also performed a phase I trial of nelarabine in 35 patients with relapsed/refractory indolent leukemias [19]. Patients were treated with nelarabine at 20, 30, 40, or 60 mg/kg by IV infusion for 1 h daily for 5 days, with nelarabine at a dose range between 1500 and 2900 mg/m2 on days 1, 3, and 5, or with nelarabine at 1200 mg/m2 on days 1, 3, and 5 combined with 30 mg/m2 of fludarabine on days 3 and 5. Pharmacokinetic data demonstrated that the dose-adjusted plasma nelarabine area under the concentration-time curve from baseline to infinity value was 59 μmol/L·h, and the half-life was 23 min. The maximum concentration of ara-G occurred at or near the end of the nelarabine infusion. The ara-G concentration increased with increasing doses of nelarabine. The dose-adjusted area under the concentration-time curve for ara-G was 521 μmol/L·h (range, 315–1177 μmol/L·h), and the half-life of ara-G was 3.7 h (range, 2.4–7.2 h). Leukemic cells isolated from the patients receiving nelarabine were analyzed for the intracellular production of ara-G triphosphate. The median peak concentration of ara-G triphosphate was 89 μmol/L (range, 22–1438 μmol/L), and the dose-adjusted median value was 65 μmol/L (range, 17–1438 μmol/L).
In a Japanese phase I study, 13 patients with relapsed/refractory T-cell acute lymphoblastic leukemia/T-cell lymphoblastic lymphoma were treated with nelarabine [20]. The maximum serum concentration of nelarabine on day 1 was 28.3 μM with the administration of 1000 mg/m2 nelarabine and 26.3 μM with the administration of 1500 mg/m2 nelarabine in adults; the half-life of nelarabine was 0.2–0.3 h. The area under the concentration-time curve of nelarabine from zero to infinity was 36.3 μM·h with the administration of 1000 mg/m2 nelarabine and 33.0 μM·h with the administration of 1500 mg/m2 nelarabine. The maximum serum concentration of ara-G on day 1 was 87.0 μM with the administration of 1000 mg/m2 nelarabine and 131.8 μM with the administration of 1500 mg/m2 nelarabine; the half-life of ara-G was 3.0–3.5 h. The area under the concentration-time curve of ara-G from zero to infinity was 440.7 μM·h with the administration of 1000 mg/m2 nelarabine and 622.5 μM·h with the administration of 1500 mg/m2 nelarabine. In children-administered 400 or 650 mg/m2 nelarabine, the maximum concentrations of nelarabine at the end of the infusions were 16.1 and 34.6 μM, respectively, and the half-lives of nelarabine were 0.2 and 0.2–0.3 h, respectively. The maximum concentrations of ara-G at the end of the 400 and 650 mg/m2 nelarabine infusions were 41.0 and 63.8 μM, respectively, and the half-life of ara-G was 1.5–2.0 h.
14.4 The Method of Administration
14.5 Toxicities
Kurtzberg et al. conducted a phase I study of nelarabine administered for five consecutive days in 93 patients with refractory hematologic malignancies [22]. The maximum tolerated dose was 60 mg/kg daily, and the dose-limiting toxicity was neurologic events. Neurotoxicity was observed in 72% of the patients; this occurred typically within 12 days of the start of drug administration in the first treatment course. The most frequent symptoms included transient somnolence, malaise, confusion, fatigue, and gait disorder, particularly in adult patients. Both central and peripheral neurotoxicities were observed. Other toxicities included nausea, vomiting, diarrhea, fever, and anorexia. As hematologic toxicities, grade 1 and 2 neutropenia and thrombocytopenia were observed.
A phase I trial of nelarabine was performed by Gandhi et al. in 35 patients with indolent leukemia, including B-cell chronic lymphocytic lymphoma and T-cell prolymphocytic leukemia [19]. The patients were treated with 20, 30, 40, or 60 mg/kg of nelarabine daily for 5 days, with 1500–2900 mg/m2 nelarabine on days 1, 3, and 5 or with 1200 mg/m2 nelarabine combined with fludarabine. Hematologic toxicity was modest. Grade 4 neutropenia and thrombocytopenia occurred in <15% of the patients. Neurotoxicities, including weakness, tiredness, drowsiness, ataxia, myalgia, and confusion, were noted, but they were transient and self-limiting. Symmetric sensorimotor peripheral neuropathy complications occurred in 21% of the patients.
Gökbuget et al. conducted a single-arm phase II study of nelarabine in 126 patients with T-cell acute lymphoblastic leukemia/T-cell lymphoblastic lymphoma [23]. Nelarabine was administered as a 2 h infusion at 1500 mg/m2/day on days 1, 3, and 5. Toxicity was evaluated in 201 cycles administered to 126 patients. As hematologic toxicities, grade 3–4 leukopenia, granulocytopenia, and thrombocytopenia were observed after 41%, 37%, and 17% of the cycles, respectively. Increases in the levels of transaminases and bilirubin were observed after 6–8% and 3% of the cycles, respectively. Neurologic toxicities were found in 20 patients (16%); the majority was grade 1–2 neurologic toxicities. Seven percent of the patients presented with grade 3–4 neurotoxicities, among which dizziness and mood alterations were the most frequent. Other toxicities included cognitive disturbance, confusion, memory impairment, and neuropathy.
A Japanese phase I study evaluated the administration of nelarabine in patients with relapsed/refractory T-cell acute lymphoblastic leukemia/T-cell lymphoblastic lymphoma [20]. A total of 13 patients (7 adults, 6 children) were studied. The most frequently encountered events were somnolence and nausea in the adults and leukocytopenia and lymphopenia in the children. Neurotoxicities, including somnolence and headache, were observed in all of the 7 adults and in 2 children.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree