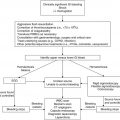
High risk (>90 % frequency of emesis in the absence of prophylaxis)
Altretamine
bCytarabine 3 g/m2/dose
Procarbazine (oral)
bCarboplatin
Dacarbazine
Streptozocin
Carmustine >250 mg/m2
bDactinomycin
bThiotepa ≥300 mg/m2
bCisplatin
Mechlorethamine
bCyclophosphamide ≥1 g/m2
bMethotrexate ≥12 g/m2
Moderate risk (30–90 % frequency of emesis in absence of prophylaxis)
Aldesleukin >12–15 million IU/m2
Cytarabine >200 mg to <3 g/m2
Lomustine
Amifostin >300 mg/m2
Daunorubicin
Melphalan >50 mg/m2
Arsenic trioxide
bDoxorubicin
Methotrexate ≥250 mg to <12 g/m2
Azacitidine
Epirubicin
Oxaliplatin >75 mg/m2
Bendamustine
Etoposide (oral)
Temozolomide (oral)
Busulfan
Idarubicin
Vinorelbine (oral)
bCarmustine ≤250 mg/m2
Ifosfamide
bClofarabine
Imatinib (oral)
bCyclophosphamide <1 g/m2
bIntrathecal therapy (methotrexate, hydrocortisone and cytarabine)
Cyclophosphamide (oral)
Irinotecan
Low risk (10–30 % frequency of emesis in the absence of prophylaxis)
Amifostine ≤300 mg/m2
Fludarabine (oral)
Paclitaxel
Bexarotene
5-Fluorouracil
Paclitaxel-albumin
bBusulfan (oral)
Gemcitabine
Pemetrexed
Capecitabine
Ixabepilone
Teniposide
Cytarabine ≤200 mg/m2
Methotrexate >50 to <250 mg/m2
Thiotepa >300 mg/m2
Docetaxel
Mitomycin
Vorinostat
Doxorubicin (liposomal)
Mitoxantrone
Etoposide
Nilotinib (oral)
Minimal risk (<10 % frequency of emesis in the absence of prophylaxis)
Alemtuzumab
Erlotinib
Rituximab
Alpha interferon
Fludarabine
Sorafenib
Asparaginase (IM or IV)
Gefitinib
Sunitinib
Bevacizumab
Gemtuzumab ozogamicin
Temsirolimus
Bleomycin
Hydroxyurea (oral)
Thalidomide
Bortezomib
Lapatinib
Thioguanine (oral)
Cetuximab
Lenalidomide
Trastuzumab
Chlorambucil (oral)
Melphalan (oral low dose)
Valrubicin
Cladribine
Mercaptopurine (oral)
Vinblastine
Dasatinib
Methotrexate ≤50 mg/m2
Vincristine
Decitabine
Nelarabine
Vinorelbine
Denileukin diftitox
Panitumumab
Dexrazoxane
Pentostatin
Recent data suggest that the frequency and severity of delayed CINV are often underestimated and remain a significant problem for patients (Dupuis et al. 2010). In order to properly manage both acute and delayed symptoms, appropriate antiemetic prophylaxis should cover the entire duration of days that symptoms are anticipated. Furthermore, for multi-agent chemotherapy regimens, antiemetic choices should be determined based on the chemotherapeutic agent with the highest emetogenic risk.
10.2.4 Classes of Antiemetics
The basis for antiemetic therapy is the neurochemical control of vomiting. Many antiemetics act by competitively blocking receptors for these substances, thereby inhibiting stimulation of peripheral nerves at the CTZ and possibly the EC. Most drugs with proven antiemetic activity in children are categorized into five groups which are discussed individually below: (1) dopamine receptor antagonists, (2) corticosteroids, (3) 5-HT3 receptor antagonists, (4) neurokinin-1 receptor (substance P) antagonists, and (5) cannabinoids. Antihistamines such as diphenhydramine which affect histaminergic receptors in the CTZ are widely utilized but have not been systematically studied. Similarly, anticholinergics, especially scopolamine, are utilized but have not been studied specifically in CINV.
10.2.4.1 Dopamine Receptor Antagonists
There are three classes of dopamine receptor antagonists effective in the prevention and treatment of CINV: phenothiazines, butyrophenones and benzamide. The most commonly used phenothiazine is prochlorperazine and has efficacy in all classes except in highly emetogenic chemotherapy (Moertel et al. 1963). A newer agent, metopimazine has been utilized with benefit in adult patients but has not been studied in children (Croom and Keating 2006; Dupuis et al. 2013). Butyrophenones, such as the antipsychotic drug haloperidol, are infrequently used in the pediatric setting secondary to their side effect profile. Of the benzamides, metoclopramide is the best studied and most widely used in children with CINV (Roila et al. 2006). Metoclopramide blocks central and peripheral D2 dopaminergic receptors at low doses and exhibits weak 5-HT3 inhibition at high doses. It is also known to speed gastric emptying and increase sphincter tone at the gastroesophageal junction. Prior to the introduction of 5-HT3 antagonists, a combination of high-dose metoclopramide and dexamethasone was the most effective prophylaxis for highly emetogenic chemotherapy (Moertel et al. 1963). Extrapyramidal effects including dystonia, tardive dyskinesia, and neuroleptic malignant syndrome (uncommon) may be seen with benzamides and thus they are not typically first-line agents (Terrin et al. 1984; Allen et al. 1985). If given for breakthrough CINV, high-dose metoclopramide at a dose of 1 mg/kg q4–6 h is typically administered in conjunction with diphenhydramine to decrease the risk of extrapyramidal symptoms (Marshall et al. 1989; Koseoglu et al. 1998). Pediatric guidelines recommend an initial metoclopramide dose of 1 mg/kg followed by 0.075 mg/kg PO q6 h for moderately emetogenic chemotherapy as a strong recommendation with minimal evidence (Dupuis et al. 2013).
10.2.4.2 Corticosteroids
Steroids, most commonly dexamethasone, are effective in preventing CINV when used alone or in combination with other antiemetic agents for all emetogenic classes of chemotherapy. The antiemetic mechanism of action is not fully understood, but they may inhibit prostaglandin synthesis in the brain (Weidenfeld et al. 1987). Clinically, steroids quantitatively decrease or eliminate episodes of CINV and may improve mood, though can also induce anxiety and insomnia.
Steroids should be given before chemotherapy for acute CINV and may or may not be repeated. Both dexamethasone and methylprednisolone have good efficacy in the prevention of acute CINV in children and are superior to low-dose metoclopramide and phenothiazines with few side effects with short-term use (Mehta et al. 1986). Dosages and administration schedules vary. Dexamethasone is also used orally for delayed CINV. Long-term corticosteroid use is inappropriate and may cause substantial morbidity. As previously shown with metoclopramide, numerous studies have demonstrated that dexamethasone potentiates the antiemetic properties of 5-HT3-blocking agents and NK1-blocking agents (Hesketh 1994; Hesketh et al. 2003; Gore et al. 2009; Choi et al. 2010). The combination of dexamethasone and ondansetron has been most studied and is recommended for first-line therapy in children receiving moderately or highly emetogenic chemotherapy; the combination of a 5-HT3 antagonist with dexamethasone has been shown to be more efficacious than a 5-HT3 antagonist alone (Dupuis et al. 2013). In one study, the “complete protection” rates increased from 43 % with ondansetron alone to 75 % with the combination of ondansetron and dexamethasone when given prior to high-dose cytarabine (Holdsworth et al. 2006). Dexamethasone may be particularly useful in patients who have demonstrated intolerance to other antiemetics given with high-dose chemotherapy or in patients with breakthrough, refractory or delayed CINV (Alvarez et al. 1995; Holdsworth et al. 2006).
Special considerations must be made prior to choosing corticosteroids for antiemetic therapy. Steroids cannot be given for CINV if the patient receives steroids as part of their chemotherapeutic regimen, as in leukemia or lymphoma. They are also typically avoided in patients with CNS malignancies, as dexamethasone has been shown to inhibit the influx of chemotherapy into the brain in preclinical models by “sealing” the blood-brain barrier (Straathof et al. 1998). Attention to protocol guidelines should be made prior to dexamethasone initiation particularly in hematologic and CNS malignancies but also potentially in protocols that utilize immunologic and biologic therapies.
Dexamethasone dosing for CINV is not well-studied. The most current pediatric CINV guidelines developed by the Pediatric Oncology Group of Ontario (POGO) suggest that dexamethasone should be dosed at 6 mg/m2 IV/PO q6 h for highly emetogenic chemotherapy (Dupuis et al. 2013). For moderately emetogenic chemotherapy, dexamethasone can be dosed at 2 mg IV/PO q12 h for patients ≤0.6 m2 and 4 mg q12 h for patients >0.6 m2. If given concurrently with aprepitant, guidelines suggest reducing dexamethasone doses by half (Dupuis et al. 2013).
10.2.4.3 5-HT3 Receptor Antagonists
Four serotonin receptor antagonists—ondansetron, granisetron, dolasetron and palonosetron—are available in the United States. Tropisetron is only available internationally and has not yet been approved by the Federal Drug Administration (FDA). When first approved in the early 1990s, these agents revolutionized the antiemetic prophylaxis of highly and moderately emetogenic chemotherapy, largely replacing phenothiazines and benzamides as first-line therapy due to superior efficacy and significantly fewer side effects (Grunberg et al. 2010). 5-HT3 receptor antagonists are thought to prevent CINV by inhibiting serotonin, which is released from enterochromaffin cells in the gastrointestinal mucosa, from initiating afferent transmission to the CNS via vagal and spinal sympathetic nerves (Jordan et al. 2007). They may also work by blocking serotonin stimulation at the CTZ and other CNS structures. Prior to 2003, there were three FDA-approved 5-HT3 receptor antagonists: ondansetron, granisetron and dolasetron. Numerous clinical trials demonstrated their clinical equivalence and showed there was no significant difference whether given orally or intravenously (Corapcioglu and Sarper 2005; Dupuis et al. 2013).
Ondansetron
Ondansetron is highly effective in controlling acute emesis induced by moderately and highly emetogenic chemotherapy in pediatric oncology patients (Carden et al. 1990; Hewitt et al. 1993). Used alone, ondansetron is superior to combination therapy with metoclopramide and dexamethasone or chlorpromazine and dexamethasone and is free of extrapyramidal and sedative side effects (Dick et al. 1995; Jimenez et al. 1997; Koseoglu et al. 1998). Ondansetron used in combination with corticosteroids has been shown superior to ondansetron monotherapy in control of CINV with highly emetogenic chemotherapy in pediatric cancer patients (Alvarez et al. 1995; Roila et al. 1998). Alvarez et al. (1995) conducted a small but significant double-blind, placebo-controlled, randomized crossover trial comparing ondansetron and dexamethasone to ondansetron and placebo in 25 children 3–8 years of age receiving highly emetogenic chemotherapy for solid tumors. Complete emetic control was achieved in 61 % receiving both ondansetron and dexamethasone versus 23 % in those treated with ondansetron alone (p = 0.04).
The lowest fully effective dose of ondansetron is 0.45 mg/kg/day, with a single daily dose schedule no less efficacious than a multiply divided dose schedule (Sandoval et al. 1999). Oral and intravenous (IV) drug administration has also been found statistically equivalent (White et al. 2000). White et al. (2000) conducted a double-blind, parallel-group, multicenter study comparing the efficacy and safety of IV and liquid ondansetron (both arms with oral dexamethasone) in the prevention of CINV in pediatric patients receiving moderately to highly emetogenic chemotherapy. Complete control of emesis was achieved in 89 % of patients in the IV group and 88 % of patients in the oral syrup group during the worst day of chemotherapy treatment and was well tolerated in both arms (White et al. 2000). For highly emetogenic chemotherapy, the recommended dose of ondansetron is 0.15 mg/kg 30 min prior to initiation of chemotherapy and repeated q8 h, although other regimens (such as 0.45 mg/kg as a single daily dose) have been shown equally effective (Dupuis et al. 2013). When giving multiple-daily dosing, ondansetron can potentially be spaced to q12 h for moderately emetogenic chemotherapy (Dupuis et al. 2013). A single-center retrospective chart review has reported ondansetron-loading doses of 16 mg/m2 (maximum, 24 mg) IV, followed by two doses of 5 mg/m2 q8 h, to be safe in infants, children and adolescents (Hasler et al. 2008).
Currently, the oral and injectable ondansetron formulations are approved for use without dosage modification in patients >4 years, including patients with renal insufficiency. Ondansetron clearance is diminished in patients with severe hepatic insufficiency; therefore, such patients should receive a single injectable or oral dose ≤8 mg. The major adverse effects include: headache, constipation or diarrhea, fatigue, dry mouth and electrocardiographic (ECG) abnormalities including QTc prolongation (Culy et al. 2001). Buyukavci et al. (2005) monitored ECGs in 22 children with acute lymphoblastic leukemia randomized to receive a single dose of either ondansetron (0.1 mg/kg) or granisetron (40 mcg/kg). The granisetron group demonstrated a significant decrease in mean heart rate at 1 and 3 h post dosing and a significant QTc prolongation at 1 h post dosing although all values eventually returned to baseline; no significant changes were seen in the ondansetron group (Buyukavci et al. 2005). Pinarli et al. (2006) randomized 38 children to either ondansetron or granisetron, and patients were monitored with a 24-h ECG post antiemetic administration. Compared to baseline, patients who received granisetron (but not ondansetron) demonstrated a significant prolongation of the QTc interval and shortening of the PR interval and QRS complex, though none of these abnormalities were clinically significant (Pinarli et al. 2006).
Granisetron
Granisetron has demonstrated efficacy in preventing and controlling CINV due to moderately to highly emetogenic chemotherapy in children and when used alone is superior to combination therapy using metoclopramide and promethazine, metoclopramide and dexamethasone, and chlorpromazine and dexamethasone (Hählen et al. 1995; Komada et al. 1999). In a small study, Hirota et al. (1993) showed no significant difference between the combination of granisetron and methylprednisolone compared with granisetron alone in pediatric oncology patients. Effective granisetron doses in children range from 20 to 40 mcg/kg/day, often administered IV once daily prior to chemotherapy or orally q12 h (Dupuis et al. 2011). In the United States, granisetron injection, transdermal patch, and oral tablets are approved for initial and repeat prophylaxis in patients receiving emetogenic chemotherapy, including high-dose cisplatin. Granisetron is pharmacologically and pharmacokinetically distinct from ondansetron; however, clinically it appears equally efficacious and safe (Gebbia et al. 1994). Current pediatric guidelines recommend granisetron at 40 mcg/kg IV as a single daily dose for moderately to highly emetogenic chemotherapy or 40 mcg/kg/dose PO q12 h for prevention of CINV from moderately emetogenic chemotherapy (Dupuis et al. 2013).
Palonosetron
A second-generation 5-HT3 receptor antagonist palonosetron was FDA approved in 2003 and boasts advantages over first-generation 5-HT3 receptor antagonists including a higher binding affinity to the 5-HT3 receptor and longer elimination half-life (i.e., 40 h in adults versus 4–8 h for first-generation agents). Large drug company sponsored adult trials have demonstrated at least non-inferiority and potential superior control of acute emesis with single-dose palonosetron compared with single-dose ondansetron or dolasetron (Eisenberg et al. 2003; Gralla et al. 2003). Gralla et al. (2003) randomized 570 adult patients receiving moderately emetogenic chemotherapy to either 0.25 or 0.75 mg palonosetron or 32 mg ondansetron on the first day of chemotherapy and showed a significantly increased prevention of acute CINV (81 % vs. 68.8 %, p = 0.009) for the 0.25 mg palonosetron arm compared with ondansetron. Of note, there was no significant difference in response to acute nausea comparing 0.75 mg palonosetron and 32 mg of ondansetron (Gralla et al. 2003). Eisenberg et al. (2003), on the other hand, showed only non-inferiority of both 0.25 and 0.75 mg palonosetron as compared with 100 mg of dolasetron. Although both studies showed significant improved response in delayed CINV for palonosetron compared with single-dose ondansetron as a secondary outcome measure, this conclusion is confounded by the inappropriate utilization of single-dose (with a 4–8 h half-life) ondansetron as a comparison dosing schedule for the prevention of delayed CINV (Eisenberg et al. 2003; Gralla et al. 2003). In a meta-analysis of adult studies of palonosetron in CINV, Likun et al. (2011) showed a significant benefit to 0.25 and 0.75 mg palonosetron in acute, delayed, and overall CINV prevention compared with first-generation agents although due to the methodologic concerns described above, it is difficult to conclude the superiority of palonosetron in delayed and overall control of CINV. However, Geling and Eichler (2005) have shown in a meta-analysis of adult patients that first-generation 5-HT3 antagonists may not be effective in the prevention of delayed CINV no matter the dosing schedule. Palonosetron’s three- to fourfold increased cost versus ondansetron must be weighed with a need for significantly less total doses (Geling and Eichler 2005; De Leon 2006; Likun et al. 2011).
Only two studies of efficacy of palonosetron in children have been published. A randomized trial of 60 pediatric patients 2–17 years of age showed 3 mcg/kg (maximum dose 0.25 mg) and 10 mcg/kg (maximum dose 0.75 mg) of palonosetron were well tolerated and equally effective (Kadota et al. 2007). Sepulveda-Vildosola et al. (2008) conducted a randomized comparison of palonosetron (0.25 mg single dose 30 min before chemotherapy) and ondansetron (8 mg/m2 every 8 h beginning 30 min before chemotherapy) in children 2–15 years, evaluating 50 chemotherapy courses in each arm and showing a significant reduction in emetic events and intensity of nausea during the acute phase of therapy (days 1–3) in the palonosetron group. Due to the decreased number of doses, they found that palonosetron was more inexpensive as well (Sepulveda-Vildosola et al. 2008). The study was limited by the fact that palonosetron was given as a standard dose rather than weight and age adjusted and also that determination of emesis and intensity of nausea was based on family report and therefore subject to potential inaccuracy (Sepulveda-Vildosola et al. 2008). Though palonosetron appears to be well tolerated, further research is needed to evaluate the optimal dose, cost-effectiveness and its relative efficacy in children based on the chemotherapeutic emetogenicity and in delayed CINV.
Comparison of Agents
Studies suggest that there are no major differences in efficacy or toxicity of the three first-generation 5-HT3 receptor antagonists (dolasetron, granisetron and ondansetron) in the treatment of acute CINV when used at appropriate doses (Hesketh 1994). Although these agents have been shown effective for the treatment of acute CINV, they have not demonstrated efficacy in alleviating symptoms of delayed CINV in adult patients (Hickok et al. 2003; Geling and Eichler 2005). The second-generation 5-HT3 receptor antagonist palonosetron has been approved for the control of delayed emesis for adult patients receiving moderately emetogenic chemotherapy, though definitive safety and efficacy has not been established in children and methodologic concerns exist in the comparison with first-generation agents for the treatment of delayed CINV.
The 5-HT3-receptor antagonists remain the cornerstone of prophylaxis for both moderately and highly emetogenic chemotherapy in children although a recent Cochrane review concluded that our knowledge of effective antiemetics in children with CINV is quite incomplete (Phillips et al. 2010). Despite the advent of 5-HT3 receptor antagonists, the control of acute and delayed CINV is suboptimal with highly emetogenic chemotherapeutic regimens, and there is considerable opportunity for improvement with either the addition or substitution of new agents in current regimens (Dupuis et al. 2011). Although lacking evidence, the recent pediatric guidelines suggest either ondansetron or granisetron can be given although no dose recommendation is given for granisetron and evidence is lacking to recommend doses or regimens with dolasetron or palonosetron (Dupuis et al. 2013).
10.2.4.4 Substance P Antagonists (NK1 Receptor Antagonists)
NK1 receptors are found in the nucleus tractus solitarii and the area postrema and are activated by substance P (Saito et al. 2003). Inhibitors of NK1 receptors have demonstrated beneficial antiemetic effects and represent a new target for antiemetic therapy. Aprepitant and its prodrug fosaprepitant have been shown to prevent both acute and delayed CINV from moderately to highly emetogenic chemotherapy in adults (Hesketh et al. 2003). Current Multinational Association of Supportive Care in Cancer (MASCC), European Society of Medical Oncology (ESMO), NCCN, and American Society of Clinical Oncology (ASCO) guidelines recommend the use of aprepitant in adults receiving highly emetogenic chemotherapy or those receiving a combination of anthracycline and cyclophosphamide (Basch et al. 2011; Jordan et al. 2011; Ettinger et al. 2012). When compared to ondansetron and dexamethasone alone, the addition of aprepitant has been shown to increase the rate of complete emetic control (i.e., no acute emesis or need for rescue medication) from 52–73 % in chemotherapy-naïve adults during a 5-day period after single-day cisplatin therapy (Hesketh et al. 2003). Subsequent randomized clinical trials in adults have demonstrated superior efficacy for the prevention of delayed CINV when aprepitant is added to a 5-HT3 antagonist and dexamethasone, recently summarized in a pooled analysis by Jin et al. (2012). These results led to FDA approval of aprepitant for adults in March 2003 for highly emetogenic chemotherapy and in 2006 for moderately emetogenic chemotherapy.
Studies of aprepitant in the pediatric population have been limited to retrospective reviews and case reports with the exception of one randomized controlled trial (Gore et al. 2009; Choi et al. 2010; Bauters et al. 2013). Gore et al. (2009) conducted a randomized, double-blind, placebo-controlled multicenter phase III trial studying aprepitant in adolescent patients. In addition to ondansetron and dexamethasone, patients were randomized 2:1 to receive either aprepitant or placebo. Forty-six patients from 11 to 19 years of age participated, with overall complete response rates of 28.6 % in the aprepitant group versus 5.6 % in the control group (though not significantly different). Serious adverse events were 32.1 % in the aprepitant group versus 16.7 % in the control group (not statistically significant) and pharmacokinetic data showed increased aprepitant metabolism as compared to historical adult data (Gore et al. 2009). Further study is required to understand the efficacy, appropriate dose and side effect profile with aprepitant in pediatric patients.
In an effort to balance access to an apparently effective antiemetic with the lack of pediatric dosing and safety information, some centers are administering aprepitant to children ≥12 years of age, weighing ≥40 kg and receiving highly emetogenic chemotherapy. The usual adult dose is administered in conjunction with a 5-HT3 receptor antagonist and dexamethasone for 3 days (Basch et al. 2011; Jordan et al. 2011; Ettinger et al. 2012). Based on the limited available data, pediatric guidelines recommend 125 mg of aprepitant on day 1 and 80 mg on days 2 and 3 (adult dosing) with a 5-HT3 antagonist and dexamethasone for patients ≥12 years receiving highly emetogenic chemotherapy (Dupuis et al. 2013). Although variable dosing regimens have been utilized in children <40 kg and reported to be well tolerated, optimal dosing is yet to be determined in this population (Choi et al. 2010; Bauters et al. 2013; Bodge et al. 2014).
As aprepitant is a moderate inhibitor of and a substrate for CYP3A4, drug interactions are an important consideration, and, as mentioned, the dose of concomitant dexamethasone or methylprednisolone (utilized as an antiemetic) is recommended to be halved. Multiple chemotherapy agents including etoposide, ifosfamide, imatinib, irinotecan, paclitaxel, vinca alkaloids and steroids are metabolized by CYP3A4 (Shadle et al. 2004). As such, aprepitant should be avoided in patients receiving these chemotherapeutic agents because of the potential for unintended increases in the dose intensity and toxicity of these antineoplastic agents. More complete references should be consulted regarding the nature and extent of drug interactions with aprepitant, as interactions with non-chemotherapeutic agents (e.g., warfarin, phenytoin, midazolam, carbamazepine, erythromycin, ketoconazole) have been described (Shadle et al. 2004). Additional NK1 receptor antagonists casopitant and rolapitant have been shown effective and safe in adult CINV but have not been studied in pediatric patients.
10.2.4.5 Cannabinoids
The plant Cannabis contains more than 60 different types of cannabinoids which have physiologic activity. There are two FDA-approved products for CINV: dronabinol (a synthetic isomer, –trans-Δ9-tetrahydrocannabinol) and nabilone. Cannabinoids likely exert antiemetic effects by targeting cannabinoid-1 (CB-1) and CB-2 receptors in the CNS (Abrahamov et al. 1995; Tramer et al. 2001). These agents have demonstrated modest efficacy in the prevention of acute CINV in children and are superior to low-dose metoclopramide and prochlorperazine, though with side effects which include euphoria, dizziness (i.e., postural hypotension) and hallucinations (Chan et al. 1987, Tramer et al. 2001). Dronabinol is dosed at 5 mg/m2 q6 h prn (max 15 mg/m2/dose) orally and is typically reserved for refractory patients. Pediatric guidelines recommend nabilone (<18 kg, 0.5 mg/dose PO twice daily; 18–30 kg, 1 mg/dose PO twice daily; >30 kg, 1 mg/dose PO three times daily) in patients for whom corticosteroids are contraindicated receiving moderately to highly emetogenic chemotherapy (Dupuis et al. 2013).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree