Fig. 2.1
Conformational change within β2GPI. (A) β2GPI as it circulates in plasma. (B) Binding to negatively charged phospholipids opens up β2GPI. (C) Binding of autoantibodies stabilizes β2GPI in its stretched conformation
Small-angle X-ray scattering (SAXS) experiments suggested that in solution, β2GPI adopts an S-shaped conformation with an additional buckle between DII and DIII [41]. Additional SAXS experiments show that β2GPI adopts different conformations, depending on pH, ionic strength, and certain cations. β2GPI turns out to be a flexible molecule, not constrained to a single, specific conformation; its conformation depends on interactions with its surroundings. Apparently β2GPI can adapt a number of different structural conformations that in vitro can coexist in a dynamic equilibrium. Factor H, a complement factor built up of 20 CCP domains, also adopts different domain orientations in solution with consequences for its functional activity [42, 43]. Proteins consisting of CCP domains vary their conformations, depending not only on the length and flexibility of the linker sequences between domains but also, predominantly, on interactions with their surroundings.
Redox Balance
Antiphospholipid syndrome is characterized by oxidative stress and systemic inflammation [44, 45]. The overproduction of reactive oxygen species (ROS) results in an oxidative microenvironment that exacerbates inflammation, inducing cell death and tissue damage, compromising antioxidant defence mechanisms [46]. Patients with APS have high levels of circulating pro-inflammatory cytokines interleukin-2 (IL-2), interleukin-6 (IL-6), and tumour necrosis factor (TNF), together with markers of oxidative stress and inflammation such as serum amyloid A (SAA), C-reactive protein (CRP), 8-isoprostane, and prostaglandin E2 (PGE2) [47, 48].
In vivo and under normal physiological conditions, β2GPI is produced in the liver and exists predominately in circulation in its free thiol form, which is less immunogenic than the oxidized form (Fig. 2.2). The precise role of β2GPI and its different forms are complex [49]; it is thought to act as a natural anticoagulant that mediates a range of functions including the clearance of liposomes, apoptotic bodies and microparticles [49–52].
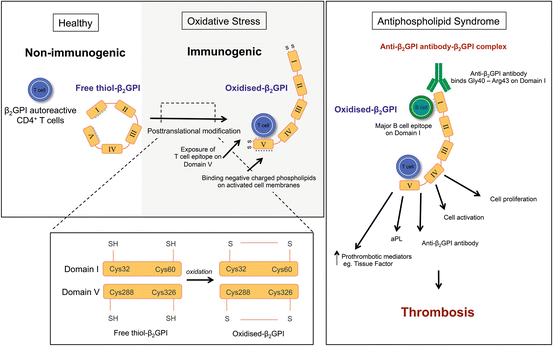

Fig. 2.2
How does oxidized β2GPI participate in the formation of thrombotic APS? During oxidative stress, free thiol β2GPI can undergo post-translational modification to form the immunogenic form, oxidized β2GPI after binding phospholipids. β2GPI autoreactive CD4+ T cells recognize newly exposed epitopes located on Domain V but not on free thiol β2GPI. A complex is formed between aβ2GPI, autoreactive CD4+ T cells and oxidized β2GPI triggering the production of aPL, specifically aβ2GPI, cell proliferation and the release of pro-inflammatory cytokines which are key events in the pathophysiology of thrombotic APS
Quantitation of Oxidized β2GPI as a Biomarker for Antiphospholipid Syndrome
Oxidized β2GPI level in APS has been proposed as a biomarker of thrombotic risk for APS. Levels of oxidized β2GPI in patients with APS are higher than in healthy subjects and patients with other autoimmune disease or non-aPL disease controls with thrombosis [53]. Free thiol β2GPI may play a protective role in APS, since free thiol β2GPI protects human umbilical vein endothelial cells (HUVEC) against hydrogen peroxide-induced cell injury [54]. Decreased plasma free thiol β2GPI may thus lower the physiological buffer against oxidative stress-induced injury. Free thiol β2GPI also protects human retinal pigment epithelium and the subretinal endothelial cell against oxidative, hydrogen peroxide stress-induced, cell death [55].
A multicentre, cross-sectional, international study using prospectively acquired samples has demonstrated that the redox status of β2GPI differs between healthy individuals and patients with thrombotic APS [53]. In the former it exists predominately with free thiols; APS patients have higher levels of total and oxidized β2GPI compared to both healthy subjects and patients with other autoimmune disease [53, 56].
Diagnostic and Prognostic Implications of the Oxidized β2GPI ELISA
Anticardiolipin antibodies, aβ2GPI, and LA test serve as diagnostic markers in APS. The predominant isotypes of aPL in APS patients are IgG aCL and IgG aβ2GPI [57, 58]. Although, non-criteria or non-classical aPL such as antiphosphatidylserine, antiphosphatidylethanolamine, and antiphosphatidylglycerol have been reported, only the three classical aPL tests are used in diagnosis of APS [59]. The LA assay identifies autoantibodies against either prothrombin and/or β2GPI, whereas the aCL assay detects the aCL and/or aβ2GPI antibodies. The aβ2GPI assay detects only antibodies against β2GPI. Concomitant triple positivity for aCL, β2GPI and LA may indicate severe APS and high recurrence risk [60], a point that is still controversial [61]. Lupus anticoagulant correlates much better with the clinical manifestations of APS than the detection of the autoantibodies with an ELISA [62, 63], and a positive LA assay due to aβ2GPI has a stronger correlation for thrombotic risk than due to antiprothrombin autoantibodies [64].
In a clinical setting, it is important to stratify risk for development of clinical events in APS and in asymptomatic, aPL-positive individuals. Delayed or inadequate treatment can result in damage and impaired quality of life [65, 66]. Although β2GPI levels are not routinely measured in patients with APS, considering the specificity of high levels of oxidized β2GPI, measuring their levels may assist in diagnosis and prognosis. The level of oxidized β2GPI is calculated by subtracting the concentration of free thiol from total β2GPI. Using an ELISA to measure post-translational redox modifications of β2GPI (including total and free thiol β2GPI) [53] (Fig. 2.3) and β2GPI plasma levels in 359 patients (identified through an international multicentre initiative) who had either APS or other autoimmune diseases or non-APS vascular thrombosis, Ioannou et al. found that the redox state of β2GPI and its concentration in APS patients had a profile distinct from that in the various control groups.
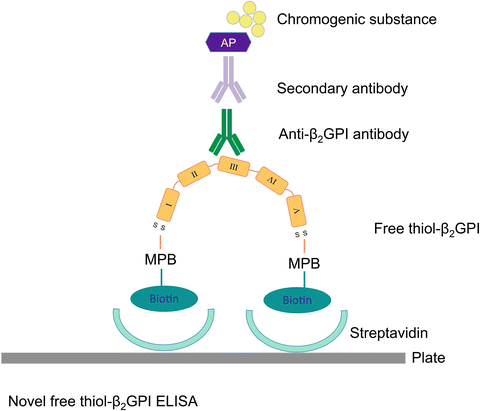

Fig. 2.3
Schema of enzyme-linked immunosorbent assay (ELISA) to measure free thiol β2 glycoprotein I (β2GPI). Free thiol β2GPI bind streptavidin via biotin-conjugated maleimidylpropionyl biocytin (MPB) and become immobilized. Acetone precipitation removes unbound MPB, and bound free thiol β2GPI is quantified with aβ2GPI and detected using a secondary antibody, alkaline phosphatase (AP) and chromogenic substance para-nitrophenylphosphate (PnPP)
Group Conclusion
Evidence from both clinical and animal studies supports the concept that β2GPI is the main autoantigen in APS. Understanding of the pathophysiology of APS and the involvement of β2GPI and its post-translational modified forms remains incomplete; understanding the relevance of oxidized β2GPI in APS will be important. Although #aPL are a defining, hallmark feature of APS, their presence does not exclusively indicate APS nor do they stratify individuals for risk of thrombosis.
Current methods for diagnosing APS patients do not incorporate quantitation of total and free thiol β2GPI. Specific ELISAs for quantifying these parameters may enhance our diagnostic and prognostic capabilities. Prospective studies may validate measurement of oxidatively modified forms of β2GPI as biomarkers. The AntiPhospholipid Syndrome Alliance For Clinical Trials and InternatiOnal Networking (APS ACTION), an international research network that collects patient samples from 25 centres around the world [67], may allow a longitudinal study that measures oxidized β2GPI.
References
1.
2.
3.
Zhou S, Chen G, Qi M, et al. Gram negative bacterial inflammation ameliorated by the plasma protein Beta 2-glycoprotein I. Sci Rep. 2016;6:33656.CrossRefPubMedPubMedCentral
4.
5.
6.
7.
Arad A, Proulle V, Furie RA, Furie BC, Furie B. β2-glycoprotein-1 autoantibodies from patients with antiphospholipid syndrome are sufficient to potentiate arterial thrombus formation in a mouse model. Blood. 2001;117:3453–9.CrossRef
8.
9.
10.
Pericleous C, Ruiz-Limón P, Romay-Penabad Z, et al. Proof-of-concept study demonstrating the pathogenicity of affinity-purified IgG antibodies directed to domain I of β2-glycoprotein I in a mouse model of anti-phospholipid antibody-induced thrombosis. Rheumatology (Oxford). 2015;54:722–7.CrossRef
11.
Reddel SW, Wang YX, Sheng YH, Krilis SA. Epitope studies with anti-beta 2-glycoprotein I antibodies from autoantibody and immunized sources J. Autoimmunity. 2000;15:91–6.CrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree