Highlights
- •
Subcentimeter nodules are common in CSI seminoma patients.
- •
Subcentimeter nodules were not associated with disease relapse in our cohort.
- •
No dedicated follow-up imaging is needed for incidental nodules in asymptomatic patients.
Abstract
Introduction and objective
Subcentimeter pulmonary nodules (SPN) found in clinical stage I (CS I) seminoma may be early pulmonary metastases or incidental, benign entities that may lead to patient anxiety and overtreatment. This study aims to demonstrate the incidence and natural history of SPN in CS I seminoma patients.
Methods
A retrospective study reviewing the medical records of CS I seminoma patients treated at UC San Diego Health between 2003 and 2023. Data collection included demographics, serum tumor markers (STM), imaging reports, pathologic findings, treatment records, and records of disease relapse. We described SPN as a finding either from a chest X-ray (CXR) or a computed tomography (CT) scan of the chest at the time of seminoma diagnosis, with a size <1cm. The incidence of SPN and relationship with disease relapse was explored.
Results
79 patients with CS I seminoma were included in the study, and mean follow-up time was 40 months. Our general practice is to observe all patients with stage I seminoma except under extenuating circumstances. Among them, 21 patients were found to have SPN, all which were diagnosed on CT scan of chest, resulting in an incidence rate of 26.6%. Notably, there was no statistically significant difference in the occurrence of SPN between patients with CS IA and CS IB (27.9% and 22.2%, respectively, P = 0.227). Four patients (5%) experienced disease relapse. None of the patients that had a relapse had an incidental subcentimeter nodules. Six patients received adjuvant chemotherapy (CMT); 1 patient had a pulmonary nodule and did not relapse; 1 patient experienced disease relapse without nodule. 10 patients underwent adjuvant radiation (RT), with no recurrence observed despite 4 of them having nodules. Additionally, 5 patients with nodules received adjuvant CMT or RT; none recurred. 16 patients with nodules were under surveillance, none recurred.
Conclusions
The incidence of SPN in CS I seminoma patient is high. Subcentimeter nodules do not appear to be related to risk of disease relapse. Our findings suggest that patients with CS I seminoma and incidental SPN can be counseled that this is a common, clinically insignificant finding. Further validation in a larger population is necessary.
1
Introduction
Testicular germ cell tumors (TGCT) are the most common malignancy among young males. In 2023, the incidence in the United States was 6 per 100,000 person-years and the death rate was 0.3 per 100,000-person years [ ]. There are 2 histologic subtypes of TGCT: seminoma and nonseminoma germ cell tumor (NSGCT). Seminoma is the most common subtype [ ]. The treatment outcomes for clinical stage I (CS I) seminomas are highly favorable, with cure rates ranging from 99% to 100%, regardless of presence and number of risk factors [ , ]. Postorchiectomy, surveillance is considered the standard management option.
A comprehensive review revealed that the recurrence rates for CSI seminoma were 15%, 2%, and 3.7% for patients who opted for surveillance, RT, and CMT respectively. Furthermore, the median survival rates specific to the cancer were 99.7%, 99.5%, and 100% for the aforementioned treatment methods respectively [ ]. Patterns of relapse in CSI seminoma are well established with about 97% occurring in the retroperitoneum and the remainder associated with elevated hCG levels [ ]. Direct pulmonary spread therefore is rare, making incidental SPNs unlikely to represent metastatic disease.
According to the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) version 1.2023, the standard imaging for staging and follow-up is chest X-ray (CXR) or computed tomography (CT) scan of the chest [ ]. Subcentimeter pulmonary nodules (SPN) found in CS I seminoma may be early pulmonary metastases or incidental, benign entities that may lead to patient anxiety and overtreatment. Currently, there is no evidence available on the occurrence of these small nodules in CS I seminoma or their influence on the likelihood of relapse. This study aims to describe the incidence and natural history of SPN in CS I seminoma patients.
2
Methods
2.1
Study population
We performed a retrospective single center analysis of patients with germ cell tumors at University of California San Diego Health from 2003 to 2023. Institutional board review was obtained (IRB no: 460337). All surgeries and clinical care were performed by urological oncologists and medical oncologists and follow-up was conducted in accordance with current clinical management guidelines. Patients with clinical stage 1 seminomas were included in the study. We excluded patients with incomplete record of chest imaging at diagnosis and second primary cancer. Treatment decisions (surveillance, adjuvant chemotherapy or adjuvant radiotherapy) were made based on treatment guidelines and pathological risk factors in consultation with the treating physician.
2.2
Data collected
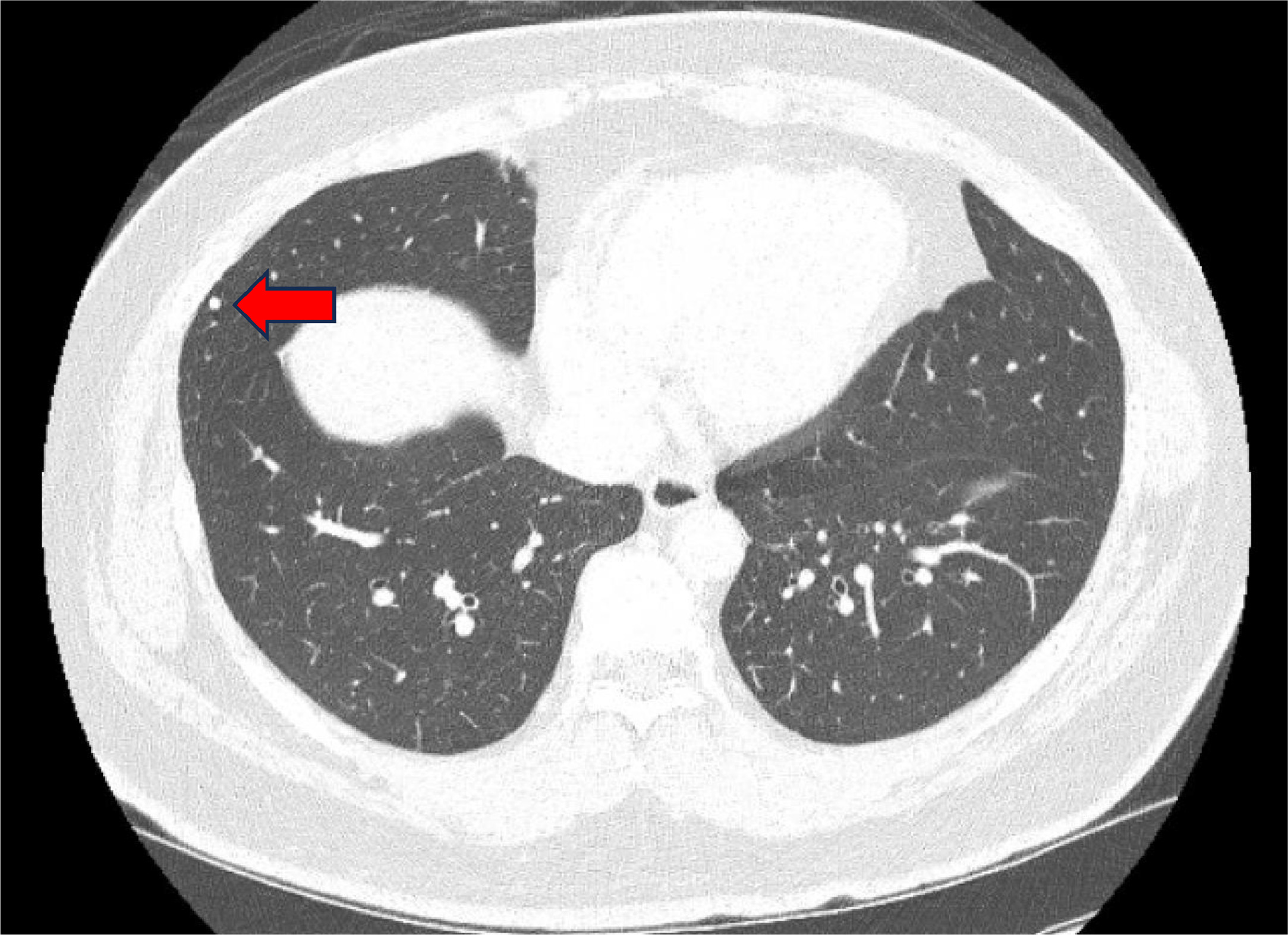
Demographic data were collected, including age, body mass index (BMI), race, alcoholic use, smoking, marijuana use, history of cryptorchidism, and family history of GCT. Clinical data were also collected, which included serum tumor markers (STM) after orchiectomy: alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), and lactate dehydrogenase (LDH), as well as imaging reports, evidence of disease relapse, and pathological findings. A SPN is defined as a finding from either a CXR or a CT scan of the chest at the time of seminoma diagnosis, with a size < 1 cm ( Fig. 1 ). The primary outcome was incidence rate of SPN in CS I seminoma patient. Secondary outcomes included risk factors of SPN and relapse rate in CS I seminoma patient.
2.3
Statistical analysis
Patients were sub-stratified by presence of a SPN on CXR or CT scan of chest at diagnosis. The 2 groups were compared with descriptive statistical analyses utilizing the independent T-test for continuous variables and Chi-square and Fisher’s exact tests for categorical variables. Nonparametric data were analyzed using the Mann-Whitney U test. The Statistical Package for the Social Sciences (SPSS) version 27 (IBM Corp., Armonk, NY, USA) was utilized for statistical analysis, with a P < 0.05 considered to indicate statistical significance.
3
Results
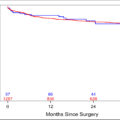
The study encompassed 79 patients diagnosed with CS I seminoma, with a median follow period of 40 months (range 12 to 240 months). Of these patients, 21 were identified to have SPNs, all of which were detected through chest CT scans at diagnosis, leading to an incidence rate of 26.6%. There were no statistical differences in age, race, ethnicity, alcoholic use, smoking, or marijuana use ( Table 1 ). The postorchiectomy STM levels were analyzed: There was no difference between AFP levels between the 2 groups (no SPN group 2.8 ng/mL, SPN group 3 ng/mL, P = 0.709). Regarding hCG levels, the median values for both groups are not significantly different, with reported values of 0 mIU/mL for the no SPN group and 0 mIU/mL for the SPN group. The p-value for hCG levels is 0.917, indicating no significant variation between the 2 groups. For LDH levels, the mean values are presented as 170.82 IU/mL for the no SPN group and 167.39 IU/mL for the SPN group. The P -value for LDH levels is 0.722, suggesting no statistically significant difference in LDH levels between the 2 groups. The pathology report demonstrates comparable tumor sizes and rates of lymphovascular invasion (LVI), tunica vaginalis invasion (TVI), intratubular germ cell neoplasia (ITGCN), rete testis invasion, hilar invasion, extra-testicular invasion, and necrosis between the 2 groups. Notably, there was no statistically significant difference in the occurrence of SPN between patients with CS IA and CS IB (27.9% and 22.2%, respectively, P = 0.227).
| Characteristics | Pulmonary nodule ( n = 21) | No pulmonary nodule ( n = 58) | P value |
|---|---|---|---|
| Age (mean ± SD) | 35.3 ± 9.5 | 38.2 ± 11.9 | 0.267 |
| BMI (mean ± SD) | 26.8 ± 4.8 | 27.5 ± 4.6 | 0.605 |
| Race | 0.927 | ||
| -American Indian/Alaska native | 0 (0.0%) | 1 (1.7%) | |
| -Asian | 1 (5.0%) | 3 (5.3%) | |
| -White | 15 (75.0%) | 40 (68.9%) | |
| -Other | 4 (20.0%) | 14 (24.1%) | |
| Alcoholic use (%) | 15 (71.4%) | 38 (65.5%) | 0.244 |
| Smoking (%) | 1/15 (6.7%) | 8/41 (19.5%) | 0.418 |
| Marijuana use (%) | 3/9 (33.3%) | 4/19 (21.1%) | 0.491 |
| History of cryptorchidism | 1/19 (5.3%) | 4/56 (7.1%) | 0.081 |
| Family history of GCT | 2/20 (10.0%) | 2/51 (3.9%) | 0.998 |
| Postorchiectomy STM | |||
| – AFP (median(Q1,Q3)) | 2.8 (1.8,4.1) | 3 (1.9,3.7) | 0.709 |
| – hCG (median(Q1,Q3)) | 0 (0,0) | 0 (0,0) | 0.917 |
| – LDH (mean ± SD) | 170.82 ± 33.6 | 167.39 ± 33.7 | 0.722 |
| Postorchiectomy STM | |||
| – AFP (median(Q1,Q3)) | 2.8 (1.8,4.1) | 3 (1.9,3.7) | 0.709 |
| – hCG (median(Q1,Q3)) | 0 (0,0) | 0 (0,0) | 0.917 |
| – LDH (mean ± SD) | 170.82 ± 33.6 | 167.39 ± 33.7 | 0.722 |
| Pathology report | |||
| -Tumor size (mean ± SD) | 4.7 ± 2.1 | 3.9 ± 1.8 | 0.123 |
| -LVI | 2 (9.5%) | 9 (15.5%) | 0.462 |
| -TVI | 1 (4.8%) | 0 (0.0%) | 0.266 |
| -ITGCN | 8 (38.1%) | 15 (25.9%) | 0.401 |
| -Rete testis invasion | 5 (23.8%) | 8 (13.8%) | 0.314 |
| -Hilar invasion | 2 (9.5%) | 6 (10.3%) | 0.211 |
| -Extra testicular invasion | 1 (4.8%) | 1 (1.7%) | 0.577 |
| -Necrosis | 3 (14.3%) | 6 (10.3%) | 0.237 |
| Clinical stage | |||
| – IA (%) | 17 (81.0%) | 44 (75.9%) | 0.227 |
| – IB (%) | 4 (19.0%) | 14 (24.1%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree