Unsolved problems
Possible solutions
Advantages/drawbacks
Uncontrolled LH surge
Flexible scheduling
Very challenging
GnRH antagonists
Additional cost, inefficient?
Low-dose clomiphene
Endometrial effect?
Premature ovulation
NSAIDs
Cheap, efficient
Empty follicle
Follicular flushing
Time consuming
Immature oocyte
In vitro maturation
Low efficiency
Mandatory single embryo transfer
Optimized ultrasound-guided embryo transfer technique
Requires training
Low per cycle efficiency
Repeated cycles
Risk of drop-out
Patient selection
Limits patients access
Pioneering Work at the Beginning of the IVF Era
Edwards and Steptoe’s initial attempts to obtain eggs for in vitro fertilization involved stimulation by hMG (150–225 IU every 2–3 day) and hCG to induce ovulation (between 1000 and 12,000 IU on days 9–11 of the menstrual cycle). Oocytes obtained this way were capable of fertilization but none of the first 77 patients who reached embryo transfer conceived successfully (although an ectopic pregnancy was obtained in 1976) [15]. Subsequently, the strategy has changed and Steptoe and Edwards had to convert to IVF in an unstimulated cycle mainly because they have noticed that the luteal phase was considerably shortened following ovarian stimulation [16]. The intensive monitoring of the natural cycle was cumbersome and involved the measuring urine excretion of oestrone glucoronide and LH levels (3 hourly), although later it was replaced by a more specific radioimmunoassay determination. They had to perform laparoscopy more than 24 h after the accumulation of LH in urine at detectable levels. Thus, the first IVF pregnancy in the world was obtained using the completely natural cycle approach [17]. Despite this seminal achievement, natural cycle IVF was technically and logistically too demanding and also inefficient to be widely adapted as a routine infertility treatment. Subsequently, it became evident that working with multiple oocyte and embryos would increase substantially the likelihood of clinical success. The next wave of practitioners developed different ovarian stimulation protocols involving clomiphene, gonadotropins, the combination of these two and with the advent of GnRH analogues the now well-established agonist (long, short and ultrashort) and antagonist protocols (multiple or single dose) were added to the armamentarium of controlled ovarian hyperstimulation [15].
Early Development of ncIVF Protocols
During the late 1980s and early 1990s, several groups tried to repeat the experience of early pioneers and establish natural cycle IVF programs but with limited success [18–20]. The Sheffield group from the UK reported extensively on its experience and outlined many of the challenges and difficulties (some of which still persist today) related to development of successful ncIVF protocols [20, 21]. The center’s protocol was a completely natural, drug-free approach without administering any stimulatory drug such as clomiphene or gonadotropins. Oocyte retrieval scheduling was based on the spontaneous LH surge only and triggering with exogenous hCG was not applied. Understandably, intensive cycle monitoring was performed requiring a lot of flexibility both from patients and the clinic’s staff. After initial attempts with semi-quantitative urine LH monitoring which was shown to be unreliable (the onset of LH surge was frequently misinterpreted due to uncontrollable changes in individual urine excretion) and also uncomfortable to patients (collecting 24 h urine samples was needed), the clinic switched to twice-daily (morning and evening sample) serum LH monitoring. For convenience, the evening (20 h) blood sample was self-obtained from capillary blood thus the patients were not required to come twice to the clinic. Hormonal monitoring usually started from cycle day 9; first with E2 determinations only, and afterward continued with measuring LH levels only in the morning samples. The onset of the LH surge was established at the first sign of increasing LH levels (>10 IU/ml), and the exact time of the oocyte retrieval was confirmed later by looking at the pattern of the last 3–4 consecutive samples. Oocyte retrievals could be scheduled between 09:00 and 17:00 daily (afternoon retrievals were needed to accommodate cycles where LH surge started in the early morning hours), and services were provided on a 7 days a week basis. Despite all these efforts in their study from the early 1990s, the authors have reported 162 treatment attempts (performed in 117 couples) of which 89 (55%) reached embryo transfer and only 9 (5.6% per started cycle) resulted in live births. The authors have argued that this relatively low success rate could be probably increased by applying more careful patient selection criteria (excluding patients >40 years and male infertility cases).
Spontaneous LH Surge Versus hCG Triggering: Which Is Better?
Whereas the risk of premature LH rise and ovulations might be diminished by GnRH antagonist co-treatment or NSAID use, successful oocyte retrieval is greatly influenced by the way of its timing. The efficiency of different oocyte retrieval scheduling strategies could be compared by looking at the “oocyte retrieval rate per started cycle” which is also influenced by cancellation rates occurring before oocyte retrieval (depending on cancellation criteria and premature ovulation rate) and the efficiency of oocyte retrieval itself (depending on center and operator-specific oocyte retrieval technique, the use of follicular flushing). An extensive review which analyzed the efficiency of ncIVF treatment in 20 studies published between 1989 and 2001 involving 1800 cycles showed that successful oocyte recovery rate varied greatly between studies (30–96%) [22]. The timing of oocyte retrieval was mostly done with exogenous hCG but with cancellation of the cycle if LH surge occurred prematurely. In the absence of an LH rise, triggering usually is performed by administering exogenous hCG at a fixed interval between 31 and 36 h. However, in some centers, oocyte retrieval scheduling timing is based on the occurrence of a spontaneous LH surge which is a far more challenging approach. In normally cycling women, ovulation is usually expected to occur 24–36 h after the onset of the LH surge (the onset of LH surge often starts in the early morning hours). However, a comprehensive study also suggested great individual variations and have found that ovulation occurred on average 41 h (range: 24–56 h) after the onset of LH surge and 18.4 h (range: 8–40 h) after the LH peak [23]. This makes the estimation of the onset of LH surge very challenging, thus only few groups planned the oocyte retrieval based on this. In the previously cited retrospective study of Zayed et al. involving 162 cycles, the oocyte retrieval rate was per planned cycle was quite acceptable with 89% [21]. A subsequent retrospective review from the same group compared the outcome of ncIVF treatments based on whether scheduling was performed according to the occurrence of spontaneous LH surge or with the use of terminal hCG injection. In the LH surge group (534 cycles), eggs were collected in 81% of the scheduled cycles whereas in the hCG group (241 cycles) this rate was slightly lower 76%. The authors concluded that compared to twice-daily LH monitoring (as described in the previous section) the administration of hCG did not have any benefit with respect of the eggs collected or pregnancies obtained [24]. In our experience (see large cohort study discussed in a later section), we routinely use LH surge-based oocyte retrieval scheduling and oocyte retrieval rates are satisfactory with 78%. Therefore, in this author’s opinion, there is currently no robust evidence which could suggest that hCG triggering is in fact superior to the spontaneous LH surge-based approach. Most clinics prefer the former approach because it permits the exact timing of oocyte retrievals (or at least gives the impression) and is convenient both for the patients and the clinic’s staff.
Recent evidence from animal models has even suggested that exposure to even small doses of hCG might have deleterious effect on endometrial receptivity [25, 26]. This is especially an issue when successive natural cycle IVF or minimal ovarian stimulation cycles are performed in a back-to-back manner or if cycles are initiated immediately after an early pregnancy loss or miscarriage. This notion was also supported by clinical studies in the context of (donor) intrauterine insemination and natural-cycle frozen–thawed embryo transfer treatment. The randomized clinical trial of Kyrou et al. on intrauterine insemination treatment showed that ongoing pregnancy rates were significantly higher (23 vs. 11%) in spontaneously versus hCG-triggered cycles [27]. Similarly the RCT of Fatemi el was stopped prematurely due to a considerable difference (31.1 vs. 14.3%) in favor of LH-triggered frozen–thawed embryo transfer cycles [28].
Modified Natural Cycle IVF Protocol: A Step Forward?
Since the advent of GnRH antagonists—beginning from the early 2000s—these drugs were also applied in the setting of ncIVF treatments, thus establishing a novel “modified” natural cycle IVF protocol. The ISMAAR classification of mild IVF approaches labeled this protocol as “semi-natural” or “controlled” cycle compared to IVF in an unstimulated or spontaneous cycle [3]. The use of GnRH antagonists in the late follicular phase was thought to be beneficial by avoiding unwanted LH surges, diminishing the risk of premature ovulation and permitting the triggering by exogenous hCG at any convenient time which could greatly simplify oocyte retrieval scheduling. In a first French pilot study, daily GnRH antagonists were administered when a leading follicle reached 12–14 mm (with corresponding E2 levels of >200 pg/ml) concomitantly with 150 IU of hMG to avoid any decline in E2 levels [29]. In a selected group of <37-year-old patients, 44 cycles were performed leading to 40 (91%) oocyte retrievals, 22 (50%) embryo transfers and five (11.4%) ongoing pregnancies. Altogether these results were encouraging, and the new protocol became the treatment of choice for many centers around the world that tried to perform IVF in a natural cycle.
In 2007, a Canadian group reported its age-specific success rates with modified ncIVF compared with conventional IVF [30]. In patients under 35 years of age, 134 cycles were performed resulting in 75 (56%) embryo transfers and 20 (14.9%) clinical pregnancies. In older patients (between 35 and 38 years), the corresponding rates were much lower 44.4 and 3.7%, respectively. In contrast with conventional IVF, success rates were considerably higher: in younger patients a 45.4% clinical pregnancy rate per started cycle was reached and in older ones it still remained quite acceptable with 33.8%. The authors concluded that although with modified ncIVF, the cancellation rate is very high it might be offset by the fact that the mild treatment option is less hard on patients, could be repeated in each month and costs less. The largest published series on modified ncIVF by Pelinck et al. reported the outcome of 1048 cycles on a cumulative basis in patients who were offered up to nine treatment cycles [13]. Per cycle ongoing pregnancy rates were 7.9% but reached 44.4% cumulatively.
Although up to date there were no direct comparisons between a completely drug-free and a GnRH antagonist-based “modified” natural cycle IVF protocol, the above studies suggest that the overall cycle outcome is basically the same as with simpler protocols that use no drugs during the follicular phase and only include the use of exogenous hCG (or GnRH agonist) for final oocyte maturation. The concept of using GnRH antagonists is intuitively appealing, but there is some evidence which suggest that antagonists cannot entirely prevent the occurrence of premature LH surges especially in the context of a non-stimulated cycles with relatively low steroid levels. Some groups even suggested that an increased (double) GnRH antagonist dose might be required to completely prevent the occurrence of LH surges even if the benefits of this approach are largely unclear, and this strategy would only contribute to increased drug costs [31]. In an innovative experimental study, Messinis et al. studies 8 women volunteers who were monitored during two menstrual cycles and submitted to transdermal E2 substitution with our without daily GnRH antagonist co-treatment [32]. The authors have found that GnRH antagonists were unable to block the occurrence of LH surge induced by supra-physiologic E2 levels. They have also suggested that GnRH antagonists behave differently during ovarian stimulation and non-stimulated cycles with an additional role of other yet unknown endocrine factors (such as the gonadotropin-surge attenuating factor).
How Efficient Is Natural Cycle IVF?
A systematic review on the efficacy of natural cycle IVF [22] was performed in 2002 summarizing the findings of 20 studies (published between 1989 and 2001) comprising a total of 1800 initiated ncIVF cycles. This review included studies where HCG was the only drug used for induction of oocyte maturation and where it was possible to calculate ongoing pregnancy rates per started cycle from published data. The review has concluded that on average, only 45.5% of initiated cycles reached embryo transfer resulting in a 7.2% ongoing pregnancy rate per initiated cycle which may partly explain the low clinical acceptance of this treatment option. However, there was a great variation among studies and embryo transfer and ongoing pregnancy rates per started cycle varied between 22.7–80% and 0–16.3%, respectively. The authors argued that (at the time of writing) future developments such as the use of GnRH antagonists for controlling LH surges or NSAID to prevent premature ovulation might further increase the efficiency of ncIVF treatments.
More recently, Roesner et al. reviewed the 5-year experience of their center in Germany involving a completely drug-free natural cycle IVF approach (hCG only) and compared it to the literature review of 28 ncIVF studies published between 1995 and 2012 [33]. In this review, they have found that clinical pregnancy rates per embryo transfer varied widely between 10.2 and 56% (unfortunately they were unable to present any data on ongoing pregnancy rate per started cycle which is a much more meaningful outcome). In comparison, this was comparable (9.8–50%) with the results published by Pelinck et al. (involving some of the same studies). Thus, it seems that during the 10-year period that separates the publication of the above-mentioned two literature reviews, there has not been any considerable improvement in the overall efficiency of natural cycle IVF treatments.
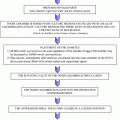
The overall low per cycle efficiency of natural cycle IVF approach can be offset by repeating successive treatment attempts. There are only two studies to date that have presented data on cumulative success rates following (modified) natural cycle approach. The earlier UK study reported the outcome 181 cycles performed in 52 infertile women [6]. Although per cycle live birth rate was only 8.8%, by performing 4 treatment attempts a cumulative live births rate of 32% was reached. The authors concluded that four cycles of ncIVF are comparable to one attempt of conventional IVF. A large-scale report form a Dutch specialist center reported the cumulative outcome of 1048 modified natural cycle IVF (mncIVF) cycles performed in 256 patients [13]. The participants (who were all <37 years of age) were offered a maximum of nine cycles (even if finally completed on average “only” 4.1 attempts). Per cycle ongoing pregnancy rates were 7.9% but reached 44.4% cumulatively. The authors concluded that modified ncIVF represents a valuable treatment alternative to conventional ovarian stimulation.
A recently published 3-year cohort study from our group also attempted to calculate cumulative success from a program based uniquely on ncIVF and minimal ovarian stimulation [34]. Although this cohort was a mixture of different mild treatment approaches, natural cycle IVF still represented 57% of all treatment attempts. Crude cumulative live birth rates were favorable in young patients (65 and 60% in <35 and <38-year-old patients, respectively), still acceptable at intermediate age (39% for patients between 38 and 40 years of age) and declined gradually in >40-year-old infertile patients. Moreover, a plateau was also detected after approximately 4–6 treatment cycles.
A recent registry-based study from the US yielded interesting insights into the efficiency of ncIVF treatment in different age groups. The review presented data on all 795 unstimulated IVF cycles that were registered in the SART (Society for Assisted Reproductive Technologies) database during 2006 and 2007 [35]. Although the total number of presented treatment cycles is significant, an important drawback of the dataset is that the average number of unstimulated cycles were <10 per year and only represented a tiny fraction (1.5%) of all treatment cycles performed at each center (therefore hardly representing the experience of specialized centers in mild IVF approaches). Natural cycle IVF was used by many clinics primarily in poor prognosis older patients with diminished ovarian reserve and poor egg quality for whom stimulated IVF was no longer an option. The authors have found that embryo transfer and live birth rates per started cycle were excellent in <35 years patients (53.8 and 15.2%, respectively), slightly lower in 35–37-year-old patients (41.6 and 14.9%, respectively), declining in 38–40-year-old patients (41.6 and 8.7%) and became considerably lower in >40-year-old patients. However, compared to stimulated IVF embryonic implantation rates were statistically superior (up until 42 years of age), suggesting improved endometrial receptivity with ncIVF treatment protocols. Thus, the authors concluded that ncIVF should be considered only in younger patients (<38 years) instead of relegating this treatment option to older patients in whom all other treatment options have failed. They have also suggested that changing the current practice of SART reporting (by reporting the outcome of unstimulated cycles separately instead of grouping them together with conventional stimulated treatment cycles) would encourage US centers to offer this treatment option more frequently.
The above findings of the US registry were also corroborated by a recent retrospective review from the Brussels group that analyzed the outcome of 469 natural cycle IVF cycles performed in 164 Bologna poor responder patients [36]. The authors have found that embryo transfer (42 vs. 59%, p = 0.011) and live birth rates (2.6 vs. 8.9%, p = 0.006) in Bologna poor responder patients were significantly lower than in controls concluding that natural cycle IVF does not represent any substantial benefit to them.
Mild IVF Approaches in Japan
There is an increasing trend in using mild IVF approaches worldwide, but these treatment modalities have become especially widespread in Japan. Data from the official Japanese ART registry shows that between 2007 and 2011 almost half (47%) of the started cycles involved unstimulated natural cycle IVF (10%) or clomiphene-based minimal ovarian stimulation (37%). This trend is probably due to a high proportion of advanced aged infertile women, the push of Japanese professional societies for the increased use of single embryo transfer and the efforts of specialist centers which have been developing these innovative treatment protocols for more than two decades [37]. One of these centers—which is also the largest single IVF unit in Japan, Kato Ladies Clinic (KLC) in Tokyo—has pioneered the development of mild IVF approaches since 1994. At KLC and at its other affiliate branches—including our center—ncIVF treatments represented a significant proportion of all cycles and considerable experience was gathered in their optimal management [38–40].
LH Surge-Based Scheduling: A Challenging Approach
In 2012, we have performed a retrospective review involving large 3-year cohort from our center with the aim of analyzing the effectiveness of oocyte retrieval scheduling based on the occurrence of spontaneous LH surge during natural cycle IVF treatment [40]. Our large retrospective study showed that in natural cycle IVF treatment oocyte retrieval timing based on the occurrence of spontaneous LH surge is feasible and permits the management of a large natural cycle IVF program on a 7-day/week basis within working hours. Moreover, acceptable oocyte recovery, fertilization and embryo cleavage rates were reached in concordance with previously published results on the efficacy of ncIVF treatment. The study included all 365 consecutive infertile patients who underwent 1138 ncIVF treatment cycles during 2008–2011 at our center (Kobe Motomachi Yume Clinic, Kobe, Japan). After obtaining informed consent, ncIVF was routinely offered to normally cycling (26–35 days) infertile women who ovulated according to their basal body temperature charts. Natural cycle IVF was usually proposed as a first, drug-free and cost-effective treatment option before starting a series of clomiphene-based minimal stimulation cycles in case no pregnancy was achieved. Patients were not selected and this treatment option was offered over a wide age range.
Natural Cycle IVF Protocol at KMYC (Kobe, Japan)
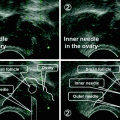
After obtaining a normal baseline ultrasound scan and hormonal profile on cycle day 3 monitoring usually started on day 8–10. Every other day follicular size was measured by two-dimensional transvaginal ultrasound scan together with serum hormonal level determinations (E2, LH and progesterone) with results available in-house within an hour. In the natural cycle IVF protocol, no GnRH antagonists were used to block the spontaneous LH surge. The center’s opening times were such that patient examination and blood collection could be performed between 08:00 and 18:30 and oocyte retrievals could be scheduled between 08:00 and 17:00 on any day of the week (the entire staff followed a 6-day/week working schedule with a variable free day). The scheduling strategy during ncIVF treatment is summarized in Table 9.2. and depicted in Fig. 9.1. When the leading follicle reached 16–20 mm with a concomitant E2 level of approximately 200–250 pg/ml oocyte retrieval was scheduled according to the presumed stage of the spontaneous LH surge. For groups 1A (pre-surge: LH <10 IU/ml) and 1B (surge start: LH between 10 and 30 IU/ml group) triggering was performed around 23:00–24:00, and OR was scheduled 2 days later in the morning (with a 30–36 h interval between triggering and OR). For triggering, a GnRH agonist was used exclusively in form of a nasal spray (busereline 600 µg) and hCG was avoided completely. With ascending LH levels (30–140 IU/ml) OR was anticipated (for the next day performed during morning or afternoon working hours) and scheduled between 15 and 31 h after the examination. The GnRH agonist triggering dose was either administered immediately after the examination (group 2: ascending slope), or in case of even higher LH levels (group 3: peak), it was omitted. In very few cases, if the spontaneous LH surge was already on its descending side by detecting increased LH with a marked decline in E2 and rising progesterone (group 4) oocyte retrieval could even be scheduled for the same day of the examination (1–2 h later). With the exception of group 4 in all subgroups low-dose, NSAIDs were systematically used every 6 h before oocyte retrieval to diminish the risk of premature ovulation. The retrospective cohort was divided in five subgroups according to the presumed stage of spontaneous LH surge on scheduling day (1A: before onset, 1B: surge start 2: ascending slope, 3: peak and 4: descending slope). In our center, oocyte retrievals could be scheduled for any day of the week between 08:00 and 17:00 h and the whole procedure usually only took 5–6 min. Transvaginal ultrasound-guided oocyte retrieval was performed without anesthesia using a very thin 21–22G needle (Kitazato Medical Co., Ltd., Tokyo, Japan)—with has virtually no dead space—hence follicular flushing was not considered useful. After oocyte retrieval, any immature (MI or GV) oocytes were observed during maximum 12 h until most of them matured spontaneously. Mature (MII) oocytes were inseminated by conventional IVF or ICSI. Normally fertilized 2PN zygotes were cultured individually in 20 μl of cleavage-stage medium until day 2 or 3 and in a majority of cases subsequently cultured from day 4–6 until blastocyst stage in water jacket small multigas incubators (Astec, Japan). Most blastocysts were vitrified electively for subsequent use in frozen–thawed blastocyst transfer cycles. Details of the vitrification method using the Cryotop® (Kitazato, Japan) were described previously [41]. Single embryo transfer was performed in all IVF treatment cycles. The procedure was performed using transvaginal ultrasound guidance by precisely placing a single embryo to the mid-uterine cavity [42]. In fresh cycles, luteal support in form of oral dydrogesterone tablets (30 mg/day) was administered during two weeks after embryo transfer and continued in case of pregnancy. Frozen–thawed embryo transfers were performed in spontaneous natural or hormonal replacement cycles [43]. Main outcome measures of this retrospective review were the rate of cycles with successful oocyte recovery, fertilized oocytes and cleaved embryos; these were compared between subgroups (1A, 1B, 2, 3 and 4). The effect of female age, infertility type, cycle rank, follicular size, serum E2 level and the presumed stage of LH surge on oocyte recovery rate was evaluated by multivariate logistic regression analysis. Additionally, overall and age-specific live birth rates were also presented. Metric variables were analyzed by one-way ANOVA test and nominal variables were analyzed by the Chi-squared test. P < 0.05 was considered statistically significant.




Table 9.2
Oocyte retrieval scheduling according to spontaneous LH surge
Group | Presumed stage of LH surge | Hormonal status on scheduling day | GnRHa triggering | Time of oocyte retrieval | Timing ranges |
|---|---|---|---|---|---|
1A-1B | Pre-surge or surge start | LH < 10 or LH: 10–30, P4 < 1.0 | 23–24:00 | 2 days later in the morning (between 08:00 and 10:00) | 30–36 h* |
2 | Ascending slope | LH: 30–53, P4 < 1.0 | Triggering immediately | 1 day later in the morning/afternoon (until 17:00) | 17–31 h** |
3 | Peak | LH: 30–140, P4 < 1.0 | Nothing | 1 day later in the morning/afternoon (until 17:00) | 15–28 h** |
4 | Descending slope | E2 and LH decreasing, P4 ≥ 1.0 | Nothing | Same day (until 17:00) | 1–2 h** |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree