The nasopharynx is the anatomical region bounded by the sphenoid bone superiorly, the soft palate inferiorly, the clivus and uppermost cervical vertebrae posteriorly, and the nasal choanae anteriorly. The lateral walls include the mucosa covering the torus tubarius, which forms the eustachian tube orifice, and the fossa of Rosenmüller, a recess that lies posterior to the torus. The eustachian tube pierces the pharyngobasilar fascia at the sinus of Morgagni, a common site for tumor infiltration. The American Joint Committee on Cancer (AJCC) defines three anatomic spaces that are in close proximity to the nasopharynx and are relevant for staging of nasopharyngeal carcinoma (NPC): the parapharyngeal space, the carotid space, and the masticator space.1 The parapharyngeal space lies lateral and posterior to the nasopharynx. It extends from the skull base down to the level of the angle of the mandible, and is anterior to the styloid process and medial to the masticator space. The carotid space is an enclosed fascial space that lies posterior to the styloid process and contains the internal carotid artery, internal jugular vein, and cranial nerves IX–XII. The masticator space includes the muscles of mastication and is enclosed by the superficial layer of the deep cervical fascia.
The lateral nasopharyngeal wall, namely the fossa of Rosenmüller, is the most common site of origin for NPC. Lateral and posterior spread into the parapharyngeal space occurs early, while invasion of the pterygoid muscles and plates occurs in more advanced disease. The degree of parapharyngeal extension has been correlated with overall survival.2 Direct tumor extension or lateral retropharyngeal lymph node metastases in the parapharyngeal space can lead to compression or invasion of several cranial nerves, including cranial nerve XII as it exits through the hypoglossal canal, cranial nerves IX to XI as they exit from the jugular foramen, and the cervical sympathetic nerves. Compression or direct invasion of the internal carotid artery can also occur in advanced disease. Anterior spread to the nasal cavity and inferior spread to the oropharynx are common and have comparable outcomes to tumor confined to the nasopharynx.3,4 In advanced cases, tumor may spread to adjacent maxillary or ethmoid sinuses. Tumor can also involve the orbital apex through the inferior orbital fissure or invade the C1 vertebral body posteriorly and inferiorly. Superiorly, tumor can invade directly through the base of skull, sphenoid sinus, and clivus. Tumor spread through the foramen lacerum provides easy access to the cavernous sinus and can lead to the involvement of cranial nerves III to VI. Perineural spread along the maxillary and mandibular branches of the trigeminal nerve can lead to intracranial extension from spread through the foramina rotundum and ovale, respectively.
The nasopharynx has a rich supply of submucosal lymphatics and has a high incidence of node involvement. At presentation, 90% of patients have clinically involved neck nodes; bilateral spread occurs in about 50% of cases, but metastasis to the contralateral nodes only is uncommon.5,6 The primary lymphatic drainage of the nasopharynx is to the lateral retropharyngeal lymph nodes and to the junctional and jugulodigastric lymph nodes (level II). The lateral retropharyngeal nodes lie near the lateral border of the posterior pharyngeal wall and medial to the carotid artery in the retropharyngeal space. The uppermost of this group of nodes is the lateral retropharyngeal node of Rouvière. The incidence of retropharyngeal lymphadenopathy ranges from 37% to 86% with clinically involved cervical lymph nodes, and from 16% to 40% for cases without clinical cervical lymph node involvement.7–9 Another direct path of spread is to the deep nodes of the posterior triangle, the spinal accessory nodes (level V). From the primary groups of nodes, further spread can occur down the jugular and posterior cervical chains. Contralateral or bilateral lymph node involvement is a result of lymphatic channels crossing the midline. Spread to the submaxillary and submental nodes occurs rarely. Spread to the parotid nodes can occur via the lymphatics of the Eustachian tube, draining to the lymphatics of the tympanic membrane and external auditory canal.
The incidence of distant metastasis at diagnosis is 3% and it may occur in 18% to 50% or more of cases during the course of the disease.10–14 Advanced neck node disease,10,15–17 particularly in the low neck,18,19 correlates strongly with the incidence of distant metastasis. The most common sites of distant metastasis are bone, followed by the lungs and liver.
The most common presenting symptom from NPC is a painless neck mass.16 The neck mass may enlarge rapidly due to necrosis or hemorrhage, and it may be tender to palpation. Other common symptoms include epistaxis, decreased hearing, nasal obstruction, pain, and cranial nerve deficits. Unilateral serous otitis media can occur as a result of obstruction, usually by compression rather than direct extension of the Eustachian tube orifice. A sore throat can occur when the tumor involves the oropharynx. Trismus results from invasion of the pterygoid muscles or involvement of the motor branches of the fifth nerve. Direct extension into the posterior orbit can result in proptosis. Pain from lifting the head and extending the neck can result from posterior invasion of the prevertebral muscles or retropharyngeal lymph nodes.
Cranial nerve involvement occurs in 12% to 24% of patients at presentation.17,20,21 Cranial nerves III to VI involvement can result from intracranial extension to the cavernous sinus, usually by tumor extension through the foramen lacerum. Lateral retropharyngeal lymph node metastases in the retroparotid space can result in involvement of cranial nerves IX to XII and the cervical sympathetic chain. Cranial nerves V and VI are most frequently involved, while cranial nerves I, VII, and VIII are rarely involved. Two syndromes can be seen in association with NPC, including the petrosphenoidal syndrome of Jacod and the retroparotid syndrome of Villaret. The petrosphenoidal syndrome presents with unilateral trigeminal neuralgia (cranial nerve V), unilateral ptosis (III), complete ophthalmoplegia (III, IV, and VI) and possible blindness (II). The retroparotid syndrome presents with dysphagia (IX and X), altered taste in the posterior third of the tongue (IX), paralysis and atrophy of the trapezius and sternocleidomastoid muscles (XI), and unilateral paralysis and atrophy of the tongue (XII). Compression of the cervical sympathetic nerve may result in Horner’s syndrome.
The recommended pretreatment evaluation is summarized in Table 54-1. Fiberoptic nasoscopes and laryngoscopes are important tools for examining the nasopharynx. Early lesions usually occur on the lateral walls but often are not visible and may only be identified as submucosal fullness in the fossa of Rosenmüller. A diagnosis of NPC is established by biopsy of a primary tumor in the nasopharynx. Fine-needle aspiration of a neck mass may establish the presence of metastatic NPC in cervical lymph nodes.
Pretreatment Diagnostic and Staging Evaluation for Nasopharyngeal Carcinoma
| History and physical examination including complete head and neck exam |
| Nasopharyngeal fiberoptic examination |
| Biopsy of the primary site or FNA of the neck |
| MRI with contrast including base of skull, nasopharynx, and neck to the clavicles |
| CT of skull base/neck with contrast as clinically indicated |
| CT scan with contrast or FDG-PET/CT of the upper mediastinum/chest as clinically indicated |
| Dental, nutritional, speech and swallowing, and audiology evaluations |
| Imaging for distant metastasis may include FDG-PET/CT and/or CT scan with contrast, especially for nonkeratinizing histology, endemic phenotype, or N2-3 disease |
| Consider EBV DNA testing |
| Consider ophthalmologic and endocrine evaluation as clinically indicated |
Magnetic resonance imaging (MRI) and computed tomography (CT) scans are the main imaging modalities for diagnosis, radiation treatment planning, and follow-up of NPC (Fig. 54-1). Compared to CT, MRI has better tissue contrast and multiplanar capacity, allowing for more accurate evaluation of the primary tumor extent.22–24 In a large series involving 420 patients, the use of MRI changed the T stage in 50% of cases and the clinical stage in 40%.25 Studies have shown that MRI is superior in detecting early skull base involvement and subtle intracranial extension.26,27 Additionally, MRI more accurately discriminates between metastatic retropharyngeal lymphadenopathy and direct tumor extension. Fluorodeoxyglucose positron emission tomography (FDG-PET) can provide valuable functional information on the extent of tumor and presence of regional lymph node involvement28,29 as well as aid in the detection of distant metastasis.30 The exact role of FDG-PET in the management of NPC is the subject of continued research.
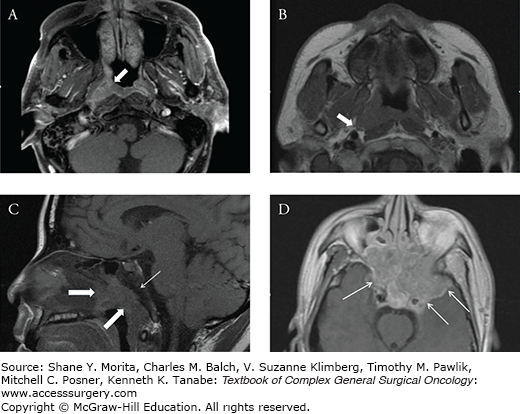
FIGURE 54-1:
A. Axial contrast-enhanced magnetic resonance imaging (MRI) scan of a patient with carcinoma of the nasopharynx, demonstrating tumor in the right fossa of Rosenmüller (arrow). B. Axial T1-weighted MRI of a patient with stage T2 carcinoma of the nasopharynx, demonstrating tumor invasion into the right parapharyngeal space (arrow). C. Sagittal T1-weighted MRI scan demonstrating a soft-tissue mass in the nasopharynx (thick arrows) with invasion into the clivus. Note the abnormal low signal intensity in the marrow of the clivus (thin arrow). D. Axial contrast-enhanced T1-weighted MRI scan demonstrating tumor invasion into the skull base and cavernous sinus (arrows).
The Eighth Edition (2016) of the American Joint Committee on Cancer (AJCC) staging system for NPC is detailed in Table 54-2 .31 It is important to understand changes to the staging system over time for interpretation of older studies which use prior classifications. The designation “T0” has been added in the Eighth Edition for an EBV-positive unknown primary with cervical lymph node involvement. Tumors confined to the nasopharynx have similar outcomes to tumors that extend to the nasal cavity and oropharynx.3,4 In the Seventh Edition (2010) system, T2a was therefore downstaged to T1, which is defined as tumor confined to the nasopharynx, or tumor that extends to the nasal cavity and/or oropharynx. Tumors with parapharyngeal involvement were classified as T2 in the 2010 system and were no longer subdivided as T2b. Tumors with parapharyngeal space involvement are at higher risk for local and regional recurrence, as well as a high rate of distant metastasis.32 In the Eighth Edition, adjacent muscle involvement (including medial pterygoid, lateral pterygoid, and prevertebral) is now designated as T2. Cranial nerve involvement has been shown to carry a worse prognosis than skull base involvement;17,18,33,34 as such, T3 disease includes involvement of the bony structures of the skull base, whereas T4 includes involvement of the cranial nerves. The previous T4 criteria of extension to the “masticator space” or “infratemporal fossa” have been replaced in the Eighth Edition by a specific description of soft tissue involvement to avoid ambiguity. NPC has a unique pattern of nodal spread as compared to other sites in the head and neck region, which is reflected in the nodal staging classification. Retropharyngeal nodes are the first echelon of nodal metastases,35 and retropharyngeal lymph node involvement independent of laterality and without cervical lymph node involvement was defined as N1 beginning in the 2010 system. Spread to the low neck correlates strongly with the development of distant metastasis.18,19 The previous N3b criterion of supraclavicular fossa extension was changed to lower neck involvement (as defined by nodal extension below the caudal border of the cricoid cartilage) in the Eighth Edition. Additionally, N3a and N3b have been merged into a single N3 category. Lastly, the previous sub-stages IVA (T4 N0-2 M0) and IVB (any T N3 M0) are now merged to form IVA, whereas the previous IVC (any T any N M1) is now upstaged to IVB.
| Definition of Primary Tumor (T) | |||
|---|---|---|---|
| T Category | T Criteria | ||
| TX | Primary tumor cannot be assessed | ||
| T0 | No tumor identified, but EBV-positive cervical node(s) involvement | ||
| T1 | Tumor confined to nasopharynx, or extension to oropharynx and/or nasal cavity without parapharyngeal involvement | ||
| T2 | Tumor with extension to parapharyngeal space, and/or adjacent soft tissue involvement (medial pterygoid, lateral pterygoid, prevertebral muscles) | ||
| T3 | Tumor with infiltration of bony structures at skull base, cervical vertebra, pterygoid structures, and/or paranasal sinuses | ||
| T4 | Tumor with intracranial extension, involvement of cranial nerves, hypopharynx, orbit, parotid gland, and/ or extensive soft tissue infiltration beyond the lateral surface of the lateral pterygoid muscle | ||
| Definition of Regional Lymph Node (N) | |||
| N Category | N Criteria | ||
| NX | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastasis | ||
| N1 | Unilateral metastasis in cervical lymph node(s) and/ or unilateral or bilateral metastasis in retropharyngeal lymph node(s), 6 cm or smaller in greatest dimension, above the caudal border of cricoid cartilage | ||
| N2 | Bilateral metastasis in cervical lymph node(s), 6 cm or smaller in greatest dimension, above the caudal border of cricoid cartilage | ||
| N3 | Unilateral or bilateral metastasis in cervical lymph node(s), larger than 6 cm in greatest dimension, and/ or extension below the caudal border of cricoid cartilage | ||
| Definition of Distant Metastasis (M) | |||
| M Category | M Criteria | ||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| AJCC PROGNOSTIC STAGE GROUPS | |||
| When T is… | And N is… | And M is… | Then the stage group is… |
| Tis | N0 | M0 | Stage 0 |
| T1 | N0 | M0 | Stage I |
| T1, T0 | N1 | M0 | Stage II |
| T2 | N0 | M0 | Stage II |
| T2 | N1 | M0 | Stage II |
| T1, T0 | N2 | M0 | Stage III |
| T2 | N2 | M0 | Stage III |
| T3 | N0 | M0 | Stage III |
| T3 | N1 | M0 | Stage III |
| T3 | N2 | M0 | Stage III |
| T4 | N0 | M0 | Stage IVA |
| T4 | N1 | M0 | Stage IVA |
| T4 | N2 | M0 | Stage IVA |
| Any T | N3 | M0 | Stage IVA |
| Any T | Any N | M1 | Stage IVB |
The incidence of NPC varies dramatically by geographic region. It is endemic in southeast China (Guangdong Province), southeast Asia, and regions of Alaska. NPC is of intermediate incidence in North Africa and in the Philippines, and is rare among Japanese and whites. The age-adjusted incidence rate (per 100,000 population per year) is 28.8 in Hong Kong, 17.2 in Eskimos, Indians, and Aleuts in Alaska, 16.8 in Singapore, 4.6 in the Philippines, 2.8 in Algeria, and 0.6 in the United States and Japan.36,37 The peak incidence of NPC occurs in the fourth and fifth decades of life, and the male-to-female ratio is 3:1.38
Epidemiologic and experimental observations suggest that the causes of NPC are multifactorial and likely related to environmental factors, viral infection, and genetics. There is a decreased incidence of NPC in successive generations of Chinese who have migrated to Western countries, though their incidence is still higher than among the indigenous populations.3,39 Environmental factors such as poor ventilation, cigarette smoking, occupational exposures, and diet have been associated with NPC. The ingestion of salted fish during early childhood or exposure to volatile nitrosamines released during cooking of salt-cured foods have been implicated as important environmental factors among the southern Chinese.38,40,41
Epstein–Barr virus (EBV) has long been associated with non-keratinizing NPC, irrespective of ethnic or geographic origin. This association is reflected by elevated EBV antibody profiles, increased circulating EBV DNA levels, and expression of the EBV genome in tumor cells.42–44 Epidemiological studies have provided substantial evidence for a hereditable component of risk for NPC.45,46 In a large case-control study from Guangdong, China, family history was the strongest predictor for NPC, with a 3.4-fold higher risk compared to the matched controls.47 Highly significant differences in histocompatibility human leukocyte antigen (HLA) patterns have been found between Chinese NPC patients and control subjects, suggesting a genetically determined susceptibility related to the presentation of viral antigens to the immune system.48
Multiple chromosomal abnormalities (copy number changes on chromosomes 3p, 9p, 11q, 12p, and 14q), gene alterations (p16 deletion and LTBR amplification), and epigenetic changes (RASSF1A and TSLC1 methylation) have been identified by various genome-wide approaches, such as allelotyping, comparative genomic hybridization, and microarray analysis.49 Investigations with precancerous lesions and in vitro immortalized nasopharyngeal epithelial cell models demonstrate evidence for the involvement of genetic alterations and EBV infection as part of the early tumorigenesis of NPC. The mutational landscape of NPC was recently characterized using whole-exome and targeted deep sequencing, as well as SNP array analysis. Integrated analysis showed enrichment of genetic lesions affecting chromatin modification, ERBB-PI3K signaling, and autophagy machinery. These results identify several candidate therapeutic targets in NPC which merit further investigation.50
Carcinomas account for 80% to 99% of the malignant tumors of the nasopharynx. Electron microscopy studies have demonstrated that the malignant epithelial cells of the nasopharynx are of squamous origin, and that undifferentiated carcinoma is a variant of squamous cell carcinoma.51,52 The World Health Organization (WHO) classification divides NPC into three histological types: squamous cell carcinoma (type 1), non-keratinizing carcinoma (type 2), and basaloid squamous cell carcinoma (type 3). Non-keratinizing carcinoma is subdivided into differentiated (type 2a) and undifferentiated (type 2b) carcinomas.53 Basaloid squamous carcinoma is a high-grade variant of squamous cell carcinoma, and rarely occurs as a primary tumor in the nasopharynx. The prior WHO terminology is widely cited in the literature and uses the following designations: squamous cell carcinoma (type I); non-keratinizing carcinoma or transitional cell carcinoma (type II); and undifferentiated carcinoma or lymphoepithelial carcinoma (type III). The term lymphoepithelial carcinoma or lymphoepithelioma is a variant of undifferentiated NPCs and describes numerous lymphocytes found among the tumor cells. The distribution of WHO histopathologic types varies with geography, race, and national origin.54 In southern China, the rates of squamous cell carcinoma, differentiated carcinoma, and undifferentiated carcinoma are approximately 3%, 9%, and 88%, while in the United States the rates are 20%, 10%, and 70%, respectively.55,56 Other uncommon malignant tumors found in the nasopharynx include adenocarcinoma, lymphoma, plasmacytoma, melanoma, and sarcomas.
The mainstay of treatment for NPC is external beam radiation therapy (EBRT). NPC has a high propensity for early cervical and retropharyngeal lymph node involvement, either unilaterally or bilaterally. Additionally, there is often infiltrative spread to adjacent soft tissue spaces, sinuses, or the skull base. Radiation therapy allows for the comprehensive coverage of both clinically apparent disease and areas at risk for subclinical spread. Several trials have demonstrated improved outcomes with sequential or concurrent chemotherapy in locally advanced NPC. Although surgery is not used in the primary management of NPC, neck dissection is utilized for persistent disease after definitive radiation treatment. Surgery can also be used in the management of local or regional recurrence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






