| Viral Adenovirus Arborvirus Chikungunya virus Enterovirus Echovirus Coxsackie A Coxsackie B Polio Flavivirus Dengue Yellow fever Hepatitis B virus Hepatitis C virus Herpesviruses Cytomegalovirus Epstein–Barr Herpes simplex Human herpesvirus 6 Varicella-zoster Human immunodeficiency virus Influenza A and B Mumps Parvovirus (especially parvovirus B19) Rabies Respiratory syncytial virus Rubeola Rubella Variola (smallpox) |
| Bacterial Actinomyces Burkholderia pseudomallei (melioidosis) Brucella Chlamydia (especially C. pneumoniae and C. psittaci) Clostridium Corynebacterium diphtheriae (diphtheria) Francisella tularensis (tularemia) Haemophilus influenzae Gonococcus Legionella pneumophila (Legionnaires’ disease) Listeria monocytogenes Mycobacterium (tuberculosis) Mycoplasma pneumoniae Neisseria meningitidis Salmonella Staphylococcus aureus Streptococcus A (rheumatic fever) Streptococcus pneumoniae Tetanus Vibrio cholerae |
| Spirochetal Borrelia burgdorferi (Lyme disease) Borrelia recurrentis (relapsing fever) Leptospira Treponema pallidum (syphilis) |
| Rickettsial Coxiella burnetii (Q fever) Rickettsia prowazekii (typhus) Rickettsia rickettsii (Rocky Mountain spotted fever) Rickettsia tsutsugamushi (scrub typhus) |
| Fungal Aspergillus Blastomyces Candida Coccidioides Cryptococcus Histoplasma Mucor species Nocardia Sporothrix schenckii |
| Protozoal Balantidium Entamoeba histolytica (amebiasis) Leishmania Plasmodium falciparum (malaria) Sarcocystis Toxoplasma gondii (toxoplasmosis) Trichinella spiralis Trypanosoma cruzi (Chagas disease) Trypanosoma brucei (African sleeping sickness) |
| Helminthic Ascaris Echinococcus granulosus Heterophyes Paragonimus westermani Schistosoma Strongyloides stercoralis Taenia solium (cysticercosis), Toxocara canis (visceral larva migrans) Trichinella spiralis Wuchereria bancrofti (filariasis) |
| Toxins Drugs Aminophylline Amphetamines Anagrelide Catecholamines Chemotherapy agents Anthracyclines Cyclophosphamide Cytarabine 5-fluorouracil Mitomycin Monoclonal antibodies Paclitaxel Tyrosine kinase inhibitors (including trastuzumab) Chloramphenicol Chloroquine Cocaine Ephedrine Ethanol Interleukin-2 Methysergide Minoxidil Phenytoin Zidovudine Environmental Arsenic Carbon monoxide Heavy metals (cobalt, copper, iron, lead) |
| Hypersensitivity reactions Drugs Allopurinol Antimicrobials Amphotericin B Azithromycin Cephalosporins Chloramphenicol Dapsone Isoniazid Penicillins Streptomycin Stibogluconate Sulfonamides Tetracycline Dobutamine Gefitinib Loop diuretics Methyldopa Mexiletine Nonsteroidal anti-inflammatories Indomethacin Mesalamine Psychiatric medications Benzodiazepines Carbamazepine Clozapine Lithium Phenobarbital Tricyclic antidepressants Thiazide diuretics Vaccines Smallpox vaccination Tetanus toxoid Venoms Insects (bee, wasp) Spider (black widow) Scorpion Snake |
| Autoimmune diseases Crohn’s disease Dermatomyositis/polymyositis Giant cell myocarditis Inflammatory bowel disease Rheumatoid arthritis Sjögren syndrome Still’s disease Systemic lupus erythematosus Systemic sclerosis (scleroderma) Takayasu’s arteritis Ulcerative colitis Wegener’s granulomatosis |
| Systemic diseases Celiac disease Churg–Strauss syndrome Collagen vascular diseases Hypereosinophilic syndrome with eosinophilic endomyocardial disease Kawasaki disease Sarcoidosis |
| Other Heat stroke Hypothermia Transplanted heart rejection Radiation |
Myocarditis can be triggered by bacterial and protozoal infections. The most common nonviral pathogens which either directly infect the heart or activate inflammatory mechanisms are Corynebacterium diphtheriae (diphtheria), Streptococcus A (rheumatic fever), Borrelia burgdorferi (Lyme disease), and Trypanosoma cruzi (Chagas disease).
Numerous medications and environmental exposures can have toxic effects on the myocardium (Table 39.2).
Pathogenesis
The pathogenesis of myocarditis in humans is not completely understood. Much of our understanding of the pathophysiology of myocarditis has been derived from murine models of enteroviral infection, particularly Coxsackievirus B3, which suggests that viral myocarditis is characterized by three stages (Figure 39.1). Stage I involves viral entry into the cardiomyocyte via endothelial cell receptors. Group B Coxsackieviruses and some adenoviruses use the Coxsackievirus-adenovirus receptor (CAR) to transport their viral genomes into myocytes. In addition to CAR, Coxsackieviruses use decay-accelerating factor (DAF) and adenoviruses use special integrins (αvß3 and αvß5) as coreceptors for viral entry. Differential binding to DAF increases viral virulence in Coxsackievirus B infections. Viral infection does not occur in the absence of CAR expression on myocytes.
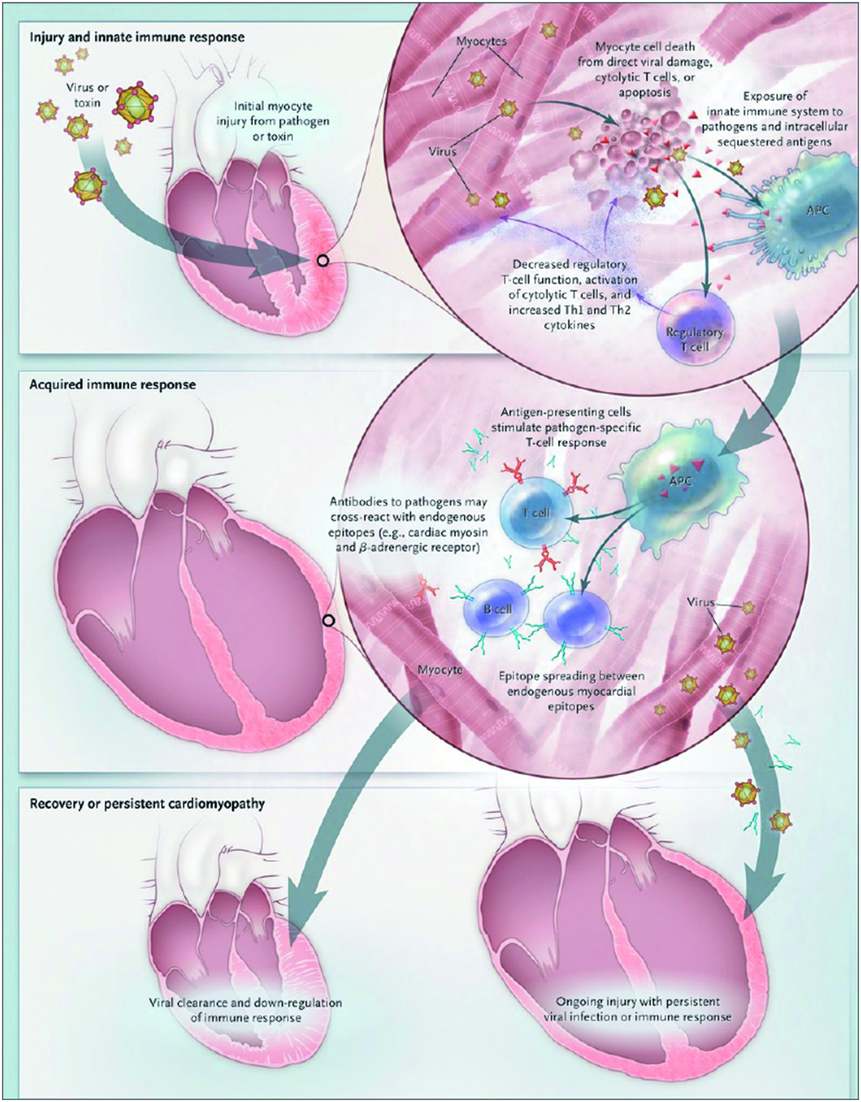
Figure 39.1 Pathogenesis of viral myocarditis
The current understanding of the pathogenesis of viral myocarditis is based on murine models. In these models, myocarditis progresses from acute injury to chronic dilated cardiomyopathy (DCM) in three distinct stages. During stage I, viral entry into cells results in direct myocardial injury, exposure of host antigens such as cardiac myosin, and activation of the innate immune system. The acquired immune response is the dominant feature in stage II, whereby activated T lymphocytes, antibodies, and autoantibodies induce significant myocardial inflammation. In most patients, stage III involves viral clearance, downregulation of the immune system, and complete myocardial recovery. In some patients, however, stage III is characterized by the persistence of viral genomes and cardiac-specific inflammation in the myocardium, leading to chronic DCM. APC = antigen-presenting cell. (From New England Journal of Medicine, LT Cooper, Jr, Myocarditis, vol 8, pp.1526–1538. Copyright (2009) Massachusetts Medical Society. Reprinted with permission.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree