Tumor
FN-Risk (%)
Regimen
Breast cancer
>20
AC docetaxel, doxorubicin/docetaxel, doxorubicin/paclitaxel, TAC
10–20
AC, EC, docetaxel, FE120C (q4 weeks), CEF
<10
CMF
Colon cancer
10–20
5-FU/folinic acid
FOLFIRI (5-FU/folinic acid/irinotecan)
<10
FOLFOX (5-FU/folinic acid/oxaliplatin)
Hodgkin lymphoma
>20
BEACOPP: bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone
Melanoma
>20
Dacarbazine-based combinations
Non-small cell lung cancer
>20
Docetaxel/carboplatin, etoposide/cisplatin
10–20
Paclitaxel/cisplatin, docetaxel/cisplatin, vinorelbine/cisplatin
<10
Paclitaxel/carboplatin, gemcitabine/cisplatin
Non-Hodgkin lymphoma
>20
CHOP (cyclophosphamide/doxorubicin/vincristine/prednisone)
DHAP (cisplatin, HD-AraC, dexamethasone)
ESHAP (etoposide, methylprednisolone, cisplatin, cytarabine)
R-CHOP (rituximab-CHOP)
Ovarian carcinoma
>20
Docetaxel, paclitaxel
10–20
Topotecan
<10
Paclitaxel/carboplatin
Small cell lung cancer
>20
ACE, ICE, topotecan
10–20
Etoposid/carboplatin, topotecan/cisplatin
<10
Paclitaxel/carboplatin
Besides the type of chemotherapy, there are patient- and tumor-specific factors influencing the risk of febrile neutropenia (Table 18.2).
Chemotherapy-related factors |
Type of chemotherapy |
Severe neutropenia with previous comparable chemotherapy |
>80 % of planned relative dose intensity |
Previous neutropenia (<1,000/μl) or lymphocytopenia |
Previous extensive chemotherapy |
Concomitant or previous radiotherapy with involvement of bone marrow |
Therapy with anthracyclines |
Mucositis of the whole gastrointestinal tract |
Patient risk factors |
Age 65 years or older |
Female gender |
Poor performance status (ECOG ≥2 “Eastern Cooperative Oncology Group”) |
Poor nutritional status |
Impaired immune function |
Tumor risk factors |
Cytopenias due to tumor bone marrow involvement |
Advanced or uncontrolled tumor |
Elevated lactate dehydrogenase (LDH) in lymphoma |
Leukemia |
Lymphoma |
Lung carcinoma |
Factors with increased risk for infections |
Open wounds |
Active infection |
Comorbidity |
Chronic obstructive lung disease |
Cardiovascular disorder |
Liver disease (elevated bilirubin, alkaline phosphatase) |
Diabetes mellitus |
Decreased hemoglobin level at diagnosis |
A review of the literature showed that higher age, especially ≥65 years, consistently correlates with a higher risk of febrile neutropenia among the independent patient-specific risk factors [1]. Higher age is an important risk factor as older patients often receive lower or even too low chemotherapy doses in fear of neutropenic complications, although those patients would benefit from an adequate dosed treatment regimen as younger patients [8].
Further independent factors with a high evidence are advanced disease, previous episodes of FN, or missing prophylaxis with G-CSF or antibiotics [1].
Many other patient- or tumor-related factors for FN are known with a lower level of evidence by retrospective analyses such as reduced general condition, impaired nutritional status, or comorbidity.
Patients with malignant diseases of hematopoiesis or lymphopoiesis have an increased risk by the disease itself and the intensity of the treatment than patients with solid tumors.
If patients older than 70 years are analyzed, then it could be shown that age alone is not a risk factor for severe or febrile neutropenia, but the type of malignancy, a planned dose intensity ≥85 %, therapy with cis-platinum or anthracyclines, previous chemotherapy, increased urea, and increased alkaline phosphatase [32].
18.5 Indication for Prophylaxis of Febrile Neutropenia with Myeloid Growth Factors According to Guidelines
Most evidence regarding the clinical effects of myeloid growth factors is derived from studies with G-CSF.
The principle of reducing neutropenia with myeloid growth factors is shown in Fig. 18.1. Neutropenia can be shortened mainly by an accelerated recovery of neutrophils.
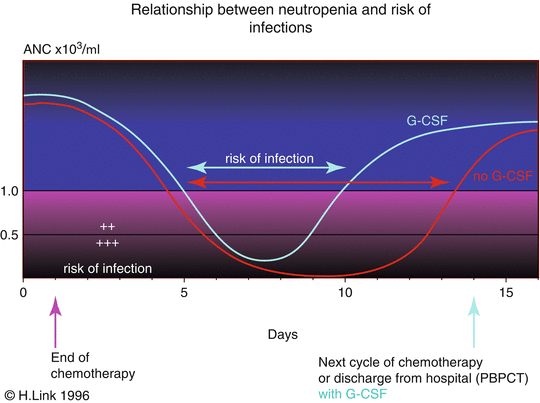

Fig. 18.1
Correlation between incidence of infections including febrile neutropenia and neutrophil recovery
The primary prophylaxis with G-CSF halves the incidence of febrile neutropenia (FN) due to chemotherapy with a risk of FN of 40 % [11, 24, 26, 34].
Primary G-CSF prophylaxis to support patients receiving cancer chemotherapy is recommended for all patients judged to be at ≥20 % risk of FN [1, 8, 33].
If using a chemotherapy regimen associated with 10–20 % FN risk, G-CSF prophylaxis should be considered based on treatment intention and individual patient risk factors. The patient’s FN risk should be reassessed prior to each cycle of chemotherapy. This is particularly important for chemotherapy regimens with 10–20 % FN risk, as patient-related risk factors may vary throughout chemotherapy cycles, and thus their FN risk could increase throughout the treatment course.
For patients at <10 % FN risk, routine G-CSF prophylaxis is not recommended.
Figure 18.2 shows the algorithm for deciding to use G-CSF after chemotherapy.
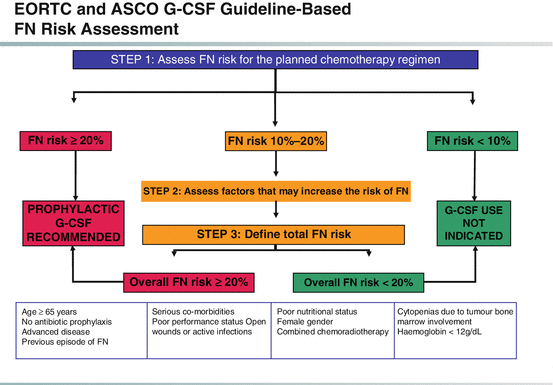

Fig. 18.2




This algorithm is a combined interpretation of the 2006 G-CSF guidelines of European Organisation for Research and Treatment of Cancer (EORTC) and American Society of Clinical Oncology (ASCO) [1, 33]. All of these organizations recommend that the physician should use their clinical judgment to assess FN risk as greater or less than 20 % according to the estimated risk of expected neutropenic complications, based on the treatment regimen and patient-specific characteristics, including age ≥65 years and experience of FN in a previous chemotherapy cycle
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree