Mycobacterium avium Complex in the Immunocompetent Host
Cassandra D. Salgado
BACKGROUND
Nontuberculous mycobacterium (NTM) disease has been reported in most developed countries with incidence rates ranging from 1.0 to 1.8 cases per 100,000 persons. Mycobacterium avium complex (MAC) organisms are the most common cause of NTM disease worldwide. These organisms are commonly found in soil and water, including both natural and treated water, as well as wild and domesticated animals. Humans are thought to acquire disease via exposure (e.g., aerosolization) to these sources. There is no evidence that NTM (MAC) is able to be transmitted from animal to human or from human to human. Lung disease is the most common clinical manifestation of NTM (MAC); however lymphatic, skin, and soft tissue, as well as disseminated disease occur. This chapter focuses on pulmonary disease in the non-HIV host.
M. avium complex includes the species M. avium and M. intracellulare. Traditional laboratory characteristics such as physical appearance and biochemical testing cannot distinguish between these species and generally speciation is not clinically relevant; however, DNA probes have been developed for identification and may be useful for epidemiologic study. M. avium is the species more often encountered among patients with disseminated disease, whereas M. intracellulare is more common in respiratory infections.
Patient-specific risk factors for NTM disease include those that predispose to disseminated disease and those that predispose to lung disease. The interactions between the organism, macrophages, and lymphocytes (particularly T helper cells and natural killer cells) are important to protect the host from mycobacterial disease. Detailed discussion of the immunologic response is beyond the scope of this brief chapter; however, interleukin (IL)-12, interferon-gamma (INF-γ), and tumor necrosis factor-alpha (TNF-α) are all important. An immunologic defect, either acquired through disease (HIV or genetic) or through receipt of immunosuppressing medications, which disrupts these pathways, may predispose to disseminated disease. Underlying structural disease predisposes to respiratory infection. This includes chronic obstructive pulmonary disease (COPD), bronchiectasis, cystic fibrosis, pneumoconiosis, and previous cavitary lung infection from tuberculosis. Additionally, it has been described that women with pulmonary MAC infections with bronchiectasis have a similar body type, sometimes including a thin build, scoliosis, pectus excavatum, and mitral valve prolapse.
IFN-γ and IL-12 protect the host from mycobacteria largely through upregulation of TNF-α and a relationship between the use of TNF-α blocking agents (such as infliximab, adalimumab, and etanercept) and development of active Mycobacterium tuberculosis has been realized among patients with a history of latent tuberculosis; however, the risk associated with receipt of these agents and NTM (MAC) infection is not known. Expert opinion suggests that patients with active NTM disease should receive TNF-α blocking agents only if they are also receiving adequate therapy for NTM.
CLINICAL DISEASE
Pulmonary Disease
Almost all patients with pulmonary MAC have a chronic or recurring cough. Some have fever, fatigue, and malaise, some have dyspnea, sputum production, and hemoptysis. The range and severity of symptoms often correlates with stage of illness (mild in early disease and more severe in advanced disease). In patients with underlying lung disease, evaluation can be difficult. The clinician should take into account changes in the patient’s baseline symptoms that cannot be otherwise explained and have a high index of suspicion for NTM. Chest auscultation may reveal rhonchi, crackles, or wheezing. NTM lung disease may present similar to tuberculosis with fibrocavitary lesions or may be characterized by nodular infiltrates associated with bronchiectasis. Thus, radiographic appearance will vary.
Fibrocavitary NTM (MAC) lung disease has been described predominately among middle-aged males who have a smoking and alcohol history. This form of disease almost always progresses within 1 to 2 years resulting in lung tissue destruction and ultimately respiratory failure. Fibrocavitary lesions tend to be thin walled with minimal surrounding parenchymal disease often with evidence of involvement of the overlying pleura. These lesions are often visible on plain chest x-ray.
Noncavitary NTM (MAC) lung disease has been described predominately among postmenopausal, nonsmoking, white females, often with the body type described above. This form of lung disease has been associated with a slower progression compared to fibrocavitary disease and appears as nodular and interstitial nodular infiltrates frequently of the right middle lobe or lingula. High-resolution computed tomography (CT) scan is needed to visualize these findings and they are described as small peripheral pulmonary nodules centered on the bronchovascular tree with cylindrical bronchiectasis often termed “tree-in bud” (Fig. 64-1).
Isolation of MAC in respiratory culture is essential for the diagnosis of lung disease; however, because the organism is ubiquitous in the environment, contamination of specimens does occur. Thus, a single positive sputum culture is often regarded as indeterminate for diagnosis. To fully optimize the utility of sputum analysis, patients should have at least three specimens collected, preferably in the morning, on separate days sent for acid-fast bacilli (AFB) staining and culture. For patients unable to produce sputum, induced sputum may be attempted but the validity of this specimen has not been established for diagnosis of NTM (MAC) lung disease. Bronchial lavage may also be useful for diagnosing NTM (MAC) lung disease and is often regarded as a more sensitive test compared to expectorated sputum. Rarely, in more difficult cases, a lung biopsy (generally transbronchial) may be needed for histopathology. Consultation with an expert experienced in diagnosing and caring for these patients is suggested.
Diagnosis of NTM (MAC) lung disease requires that the patient meet both clinical and microbiologic criteria. Clinical criteria include pulmonary symptoms, nodular or cavitary opacities on chest imaging, and exclusion of other diagnoses such as active tuberculosis or malignancy. Microbiologic criteria include positive culture results from at least two separate expectorated sputum samples, or a positive culture result from at least one bronchial wash or lavage, or a transbronchial or other lung biopsy with mycobacterial histopathologic features (granulomatous inflammation) and positive culture for NTM, or biopsy showing mycobacterial histopathologic features (granulomatous inflammation) and one or more sputum or bronchial washing that are culture positive for NTM.
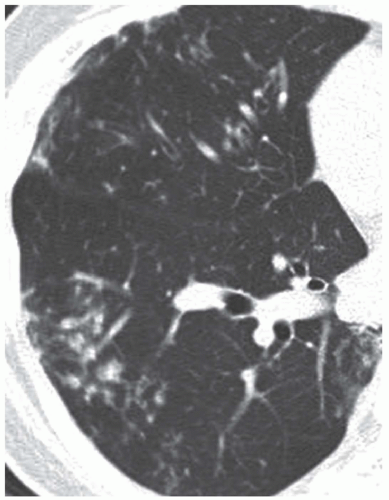 Figure 64-1 High-resolution CT Scan in a patient with MAC pulmonary disease. Bronchiectasis with centrilobular small nodules and tree-in-bud signs extending more than 2 cm from pleura, in superior segment of right lower lobe. (From Song JW, Koh WJ, Lee JY, et al. High-resolution CT findings of Mycobacterium avium-intracellulare complex pulmonary disease: correlation with pulmonary function tests. AJR Am J Roentgenol 2008;191:1010-1017, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
