Fig. 11.1
Typical osteosarcoma of left distal femur in an 8-year-old boy. An osteoblastic process permeates the medullary cavity. The periosteum is elevated, producing a Codman triangle
Soft tissue sarcomas (STS) comprise a diverse group of malignancies. About 850–900 STS are diagnosed each year in the United States, in children 0–19 years of age [4]. Rhabdomyosarcoma (RMS) is the largest diagnostic group in children. Non-RMS diagnoses include fibrosarcoma, malignant fibrous histiocytoma, leiomyosarcoma, liposarcoma, synovial sarcoma (Fig. 11.2), along with other rare types of sarcomas.
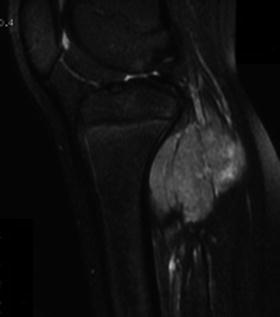

Fig. 11.2
Nine-year-old girl presented with a rapidly enlarging mass in her calf. T2-weighted MRI shows the tumor located in posterior compartment of the leg. The biopsy revealed a synovial sarcoma
Patients most commonly present with pain at the site of the primary tumor, often accompanied by a palpable mass on physical examination and a lesion on radiographic imaging. Both CT and MRI scans have been employed and provide differentiating information regarding soft-tissue spread and bone and/or joint involvement. Detailed examination of the lungs by CT scan and the bones by bone scan is required to evaluate clinically detectable metastatic lesions. Laboratory blood tests may show elevations in alkaline phosphatase (ALK), lactate dehydrogenase (LDH), or erythrocyte sedimentation (ESR). The definitive diagnosis relies on the biopsy, even when physical findings and radiographs appear pathognomonic for OS and ES. The surgeon should plan a biopsy very carefully, with consideration for subsequent definitive surgery or radiotherapy. A poorly-conceived or poorly-placed biopsy may jeopardize the subsequent treatment, especially during a limb-salvage procedure [5]. An improper biopsy can lead to tumor spread and problems removing the tumor later on. An incisional biopsy done in the wrong place can make the subsequent removal of the malignant tumor much more complicated.
Primary bone and soft tissue tumors are most commonly staged with the Musculoskeletal Tumor Society (MSTS) system devised by Enneking et al. [6] in 1980. The American Joint Committee on Cancer (AJCC) has proposed an alternative staging system based on the primary tumor, lymph node metastases, and systemic metastases [7, 8]. The specific therapeutic approaches for patients vary with the tumor pathology and the presence or absence of metastatic disease. Treatment of malignant bone and soft tissue tumors involves neoadjuvant chemotherapy for systemic control and surgery and/or radiation for local control. The major surgical procedures are amputation and limb salvage. With the advances in multimodality treatment, better understanding of tumor biology, new diagnostic imaging techniques and supportive care, the survival rate has increased dramatically since the 1970s and 1980s. Based on a recently published meta-analysis on worldwide data [9], the survival rate measured in 47,227 patients with osteosarcomas remained stable at approximately 20 % from the 1910s to the 1960s, with a sudden surge to approximately 60 % during the 1980s. Since that time, despite new innovative therapies, the survival rate has continued to remain unchanged. During this time, however, there was a shift in the surgical approach. Whereas limb amputation was the procedure of choice in the 1970s, limb salvage surgery is often a viable option today [10]. The use of chemotherapy is critical to a successful surgery and should be considered an integral part of the surgical plan.
11.2 Brief Overview of Surgical Treatment
The goal of surgery in pediatric bone and soft tissue sarcomas is curative resection and, when possible, preservation of bone growth. The ideal procedure should maximize the patient’s cosmetic and functional outcome but should not jeopardize the patient’s long-term survival. In most cases, limb salvage has obvious cosmetic advantages, provides psychological benefit as well as improved functional outcome, and avoids the needs and complications of external prosthetic devices. However, limb salvage surgery is more technical and has a higher rate of complications, including infection, nonunion, fracture, poor joint movement, leg-length discrepancy, prosthetic loosening, mechanical failures and local recurrence than amputation does [11]. These late complications can be problematic, and the perceived advantage of limb salvage can be negated by the need for additional revision surgeries, or ultimately an amputation. Amputation may be the treatment of choice in some situations, including biopsy incisions that contaminate the soft-tissue envelope, tumor involvement of the nerves and/or major blood vessels, and pathologic fracture. However, a pathologic fracture is not always a contraindication for limb salvage, and vessels can be resected and bypassed if encased by tumor. In these circumstances, the decision for limb salvage has to be made on a case-by-case basis [12].
Most of the tumors will occur in patients who are skeletally immature. The location of these tumors in the growing areas of bone commonly necessitates the removal of the involved growth plate (Fig. 11.3) [13].
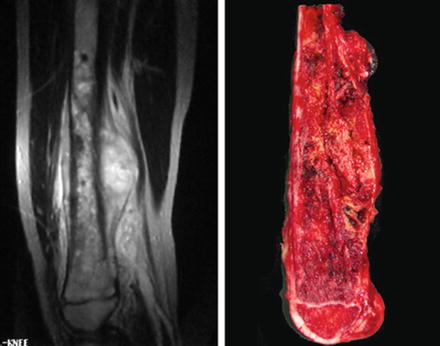

Fig. 11.3
Osteosarcoma in the distal metaphysis of the femur of an 11-year-old boy. (Left) Sagittal MRI shows the tumor is in contact with the growth plate. (Right) The specimen shows the growth plate is resected
In the growing child, limb salvage procedures present unique challenges. These include the small size of the pediatric skeleton, the growth potential of the patient, the proposed final length of the unaffected limb, and the need for correction of the ensuing limb salvage discrepancy [14]. Amputation can also produce problems in growing patients. Stump overgrowth (bone growing after an amputation in skeletally immature patients) can lead to painful pressure on the soft tissue stump after amputation, requiring revision surgery [2].
Young sarcoma survivors not only have to deal with limb function-related problems, but they also have an increased mortality because of therapy-related late effects. This chapter presents late complications commonly observed after surgical treatment for pediatric patients with musculoskeletal malignancy.
11.3 Late Local and Distant Relapse
As more pediatric cancer patients enter long-term follow-up as survivors of their cancer, it is important for both patients and physicians to appreciate the risk of late local and distant relapse. Some young adult survivors of childhood cancer eventually outgrow their pediatric care and have uncertain medical follow-up in their adulthood [15].
Most patients with musculoskeletal malignancy who develop recurrent disease do so within 2–3 years after completion of treatment. Relapse after being disease-free for 5 or more years is uncommon. Late relapse has, however, been documented in long-term follow-up of large series of patients with osteosarcoma. Vigilance must therefore be encouraged for both the patients and the multidisciplinary medical professionals who treat them. The Cooperative Osteosarcoma Study Group (COSS) published a detailed analysis of the long-term outcome of 1,702 patients with extremity and trunk osteosarcoma. With a median follow-up of 5.5 years, 23 (1.4 %) patients were found to have relapsed after 5 years, with the latest care being 14.3 years after treatment [16]. The Rizzoli data showed an incidence of late relapse of 5.5 % [17]. The latest recurrence of osteosarcoma reported was recurrence as late as 17 years from initial diagnosis and treatment [18]. Besides osteosarcoma, very late (>10 years) local recurrence of Ewing’s sarcoma has also been reported [19].
The lungs are the most common sites for the development of metastases in pediatric patients with bone and soft tissue tumors. Skeletal metastases are less common but have also been reported in the literature [20]. Patients with metastatic osteosarcoma limited to the lung, who have received a complete resection of the pulmonary disease, have reported survival rates of up to 30 % [21, 22]. There is no uniform surgical guideline on how to manage osteosarcoma pulmonary metastases. However, complete resection should be attempted, regardless of approach or operation performed, as long as the primary tumor is controlled [23]. Pulmonary metastasectomy in most cases means resecting single pulmonary nodules located near the pleura. All thoracic interventions have their place in pulmonary metastasectomy from wedge resections to laser resection and extended chest wall replacement [24]. Currently, the most common approach to the OS patient with unilateral pulmonary metastases is unilateral thoracotomy with resection, followed by close surveillance [21]. A retrospective study suggested that osteosarcoma patients with early unilateral pulmonary metastases might not benefit from exploration of the contralateral hemithorax [25].
The prognosis of patients who relapse with bone metastases is worse than the prognosis of patients who first relapse with lung metastases. The experience from Rizzoli indicated that, even if treated with aggressive surgery and chemotherapy, only 10 % of patients with OS who relapse with bone metastases actually survive [26].
These findings highlight the need for lifelong follow-up of bone and soft tissue sarcoma patients. Meticulous follow-up is required to permit early detection and successful therapeutic intervention.
11.4 Late Complications of Limb Salvage Procedure
The major surgical procedures for bone and soft tissue tumors are limb salvage and amputation. In children for whom limb salvage surgery is an option, the choice between amputation and limb salvage is not always clear. Quality of life in terms of function, psychological outcome and endpoint life span achievements such as marriage and employment apparently do not differ significantly between amputee and nonamputee osteosarcoma survivors [27, 28]. In most cases, limb salvage has an obvious cosmetic advantage, and is more acceptable to patients. However, limb salvage procedure has a higher rate of complications. It is always challenging for the musculoskeletal oncologist to make the appropriate decision in choosing between limb salvage and amputation.
Within limb salvage, there are also multiple procedures to choose from. Multiple options for limb salvage surgery have been developed over the past 30 years (Table 11.1). The choice of limb salvage procedure depends on the location and extent of tumor, psychosocial considerations, and the age of the patient. Endoprosthetic and biological reconstruction are two major types of limb salvage surgeries. There is additional challenge in the bony reconstruction of children, due to their continuing growth. The potential for further limb growth is affected when tumor removal necessitates resection of one or more growth plates. The related late complications will be discussed below.
Table 11.1
Surgical options after tumor resection in pediatric limb salvage procedure
Endoprosthetic replacement |
Allograft |
Allograft prosthetic composite (APC) |
Vascularized graft (or combined with allograft) |
Devitalized and re-implantation of tumor bone |
Bone transportation using an external fixator |
Rotationplasty |
11.4.1 Biological Reconstruction
11.4.1.1 Autograft
Both free and vascularized fibular grafts are used frequently in the reconstruction of bone defects, which occur after tumor resection in children. Fibular graft has been used most successfully in the reconstruction of upper limb bones (Fig. 11.4) [29]. Attempts have been made to maintain epiphyseal growth by harvesting the fibula in young children while maintaining the blood supply to the fibular epiphysis [30]. The fibula provides well-perfused bone and the capability of osteogenesis, but it often lacks the structural strength of allografts for lower limb reconstruction [31]. Fibular grafting is particularly attractive when considering bony reconstruction in the adolescent and pediatric population, in which long-term viability and mechanical stability are required. Long-term radiographic studies showed fibular incorporation into the allograft (Fig. 11.5) [32, 33]. Stress fracture and nonunion are two major complications after fibular reconstruction. The stress fracture of the transferred fibula was reported ranging from 12.5 % to 28.5 % [34, 31]. The nonunion or delayed union rate was reported to be 8–14 % [29, 35].
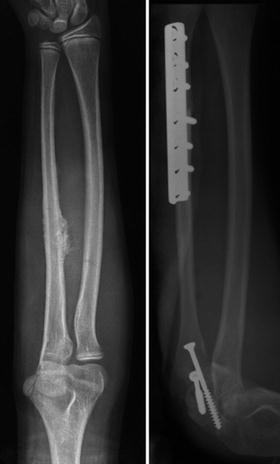
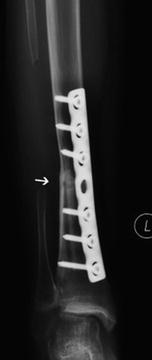

Fig. 11.4
Radiograph showing osteosarcoma of the left ulna in a 13-year-old boy. The tumor was resected and the bone defect was bridged with free fibular graft

Fig. 11.5
Osteosarcoma in the distal metaphysis of the tibia of a 9-year-old boy. Reconstruction with an osteoarticular allograft and intra-medullary vascularized fibular graft stabilized with plate after tumor resection. The incorporation of the allograft at the proximal osteotomy (arrow) was observed at 1 year after surgery
Another autograft option is to use the diseased bone after devitalization. The patients’ own tumor-bearing bone is sterilized by irradiation, microwave, pasteurization or autoclave, however, this technique will be useful only for diaphysis or flat bones in children [36]. There are very little reports on large numbers of cases with recycled tumor bone reconstruction, and the complication rates varied widely among different studies. Infection and fracture of the reimplant, though, are the main complications [37, 38].
11.4.1.2 Allograft and Allograft-Prosthesis Composite (APC)
Allograft can be used alone or in combination with prosthesis as an APC or in combination with a vascularized fibula graft. However, allografts do not have osteo-inductive function, which can induce new bone formation. Allograft can include the epiphysis (osteoarticular), replace a midsection of bone (intercalary), or be used as part of a resection arthrodesis [39–41]. Bone allograft can restore articular congruity, preserve bone stock, and provide musculotendinous attachment sites for host soft-tissue structure, theoretically improving function [41–43].
Allograft reconstruction procedures have higher rates of complication in children than adults [10]. Osteoarticular allograft has higher failure rates in the lower extremity. Intercalary allograft does better. Complications of allograft reconstruction have been well described in literature. However, there are only a few references in literature, which exclusively describe pediatric sarcoma population. Fracture, nonunion, and infection are common complications after allograft reconstruction in children (Table 11.2). The overall 5-year and 10-year allograft survival rates were 69–78 % and 63 % respectively [40, 44].
Table 11.2
Complication rates of allograft reconstruction in children
Study | Year | Number of cases | Complication | ||
|---|---|---|---|---|---|
Infection (%) | Nonunion (%) | Fracture (%) | |||
Muscolo et al. [41] | 2008 | 22 | 4.5 | 4.5 | 13.6 |
Ramseier et al. [45] | 2006 | 21 | 9.5 | – | 28.6 |
Brigman et al. [42] | 2004 | 116 | 16 | 34 | 27 |
Alman et al. [39] | 1995 | 26 | 11.5 | 11.5 | 53.8 |
Infection is a potentially devastating complication of allograft reconstruction. Surgical extent, soft tissue loss, multiple operations, and chemotherapy cause the allograft to be more susceptible to infection [44]. Union at the graft-host interface is dependent on the proliferation of host bone cell, quality of contact, and stability of the junction. Hornicek et al. analyzed the factors affecting nonunion and found that chemotherapy negatively affected union at the allograft-host junction [46]. The fracture rate in children has been reported to be significantly higher than the studies that included patients in all age groups [10, 39]. Spanning the entire length of the allograft with internal fixation theoretically splints the graft and offers protection from bending and torsional forces, which can cause fractures [47]. The mechanical failure of internal fixation can be observed in mid-to-long-term follow-up (Fig. 11.6). Leg length discrepancy is commonly seen in most patients who received allograft reconstructions secondary to loss of the growth plate. The degree of leg discrepancy depends on the age of the patients at the time of their first treatment.
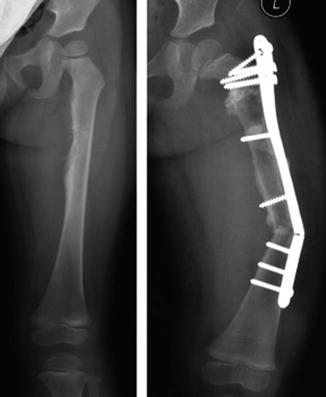

Fig. 11.6
The radiograph showing an Ewing’s sarcoma in the diaphysis of femur of a 5-year-old girl. Reconstruction was carried out with an intercalary allograft. Mechanical failure of the plate occurred 18 months postoperatively
11.4.2 Endoprosthetic Reconstruction
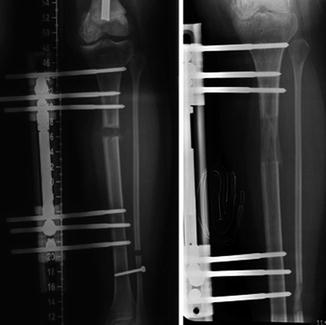
In adults, modular tumor endoprostheses, an implantable artificial metal device to replace a missing segment of bone and adjacent joint, are becoming a standard option for reconstructing the limb after resection of malignant bone tumors. However, reconstruction with the standard static prosthesis is neither a practical nor a functional option in the skeletally immature patient due to the length discrepancy [14]. In order to solve the problem in children, the concept of the expandable endoprosthesis was introduced by Scales in 1976 [48]. An expandable endoprosthesis can be lengthened as the child grows, similar to the standard endoprosthetic reconstruction. It allows immediate weight bearing with early rehabilitation and return to function. The design of expandable endoprosthesis has significantly evolved over the past 30 years (Fig. 11.7).
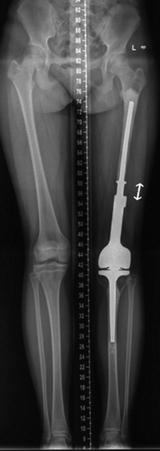

Fig. 11.7
Radiograph at a follow-up of 4 years showing 38 mm lengthening (extension arrowed) of a female patient aged 12 years at implantation of a minimal invasive expandable endoprosthesis
By then, all the lengthening mechanisms required a formal surgical procedure, which resulted in a high incidence of infection [49]. The younger the child, the more operations were needed not only for lengthening, but also for management of complications such as stiffness, infection, wear, and loosening. The latest design has a noninvasive lengthening mechanism, which relies on an electromagnet outside the body to transfer energy to the sealed implant inside the body. Today, there are several noninvasive products that have the ability to lengthen a child’s limb without having to undergo an operation [13, 36, 50, 51]. This provides a substantial advantage for both the child and the parents. Not all children are candidates for an expandable prosthesis. The size, age, and potential growth of the patients need to be evaluated carefully. Endoprosthetic reconstruction is not recommended for patients younger than 6–8 years old or for small children who have tall parents, since their potential of growth is too much to be corrected even with the use of expandable prosthesis [14, 52]. Beebe et al. [53] analyzed the functional outcome and gait of children after reconstruction with noninvasive expandable prosthesis at a mean follow-up of 31.5 months. Increased time spent in double-limb support and decreased gait velocity was observed, indicating weakness of hip abductors. However, the reconstruction produces satisfactory functional outcome and the patients maintain high levels of emotional acceptance.
Complications of expandable endoprosthesis include infection, loosening of implants, stiffness, unplanned shortening or lengthening, outgrowing the available extension, and implant and periprosthetic fracture [49]. Infection poses the largest iatrogenic risk to limb salvage. The deep infection rate is reported to be 10.9–17.6 % [54–56], most prevalent in the proximal tibia. Infection of an implant is difficult to eradicate because of the adherent colonies of bacteria in a polysaccharide matrix, collectively called a biofilm [57]. Infecting bacteria can remain on the surface of an implant for a variable length of time. Clinical symptoms can be caused by the local proliferation of bacteria shed by the biofilm, and may present themselves from several months to years after index surgery. Chronic infection usually requires a two-stage revision with a 70 % success rate, but some patients still require amputation [49]. Aseptic loosening of endoprosthesis is the most frequent complication in pediatric limb salvage, which has been reported to be 7.1–26.3 % [58, 50]. This is largely due to the long lever arm created at the bone-implant interface, and also, difficult anchoring conditions. Mechanical failure is another frequently reported complication in the literature, most frequently involving failure of the expansion mechanism. There are also reports regarding the fatigue fracture of the prosthesis [59]. The primary biological limiting factor to using an endoprosthesis in children is the diameter of the host residual shaft diameter. Under long-term cyclical loading, even strong material will fail if the stem diameter is less than 8 mm [51]. Picardo et al. reported that revision procedure was performed in 16.4 % of the cohort after the endoprosthesis was maximally extended. The revision rate due to mechanical complications was 18.2 % [54]. Despite these complications, the literature suggests the existence of a role for expandable prostheses in pediatric limb reconstruction after resection of a primary malignant bone tumor (Table 11.3).
Table 11.3
Studies describing outcomes of expandable endoprostheses in literature
Author | Year | No. of cases | Endoprosthesis | Average length of expansion (mm) | Overall complication rate (%) | Function score (MSTS) (%) |
|---|---|---|---|---|---|---|
Henderson et al. [56] | 1996–2009 | 38 | Strykera 18 | 30 | 42 | 87 |
Biometb 12 | 31 | |||||
Stanmore 8 | 81 | |||||
Saghieh et al. [60] | 2002–2009 | 17 | Wright | 25.8 | 70.6 | 90 |
Picardo et al. [54] | 2002–2009 | 55 | Stanmore | 38.6 | 29.1 | 80.7 |
Hwang et al. [55] | 2002–2009 | 34 | Stanmore | 32 | 23.5 | 85.6 |
Baumgart [61] | NA | 5 | Implantcast | 78 | 60 | NA |
Wozniak [61] | 2000–2010 | 118 | Stanmoreb 47 | NA | 31.9 | 85 |
Wright 4 | 50 | |||||
Implantcast 67 | 26.8 |
11.4.3 Leg Length Discrepancy
Limb length discrepancy remains a critical late effect after limb salvage procedure in children (Fig. 11.8) [14].
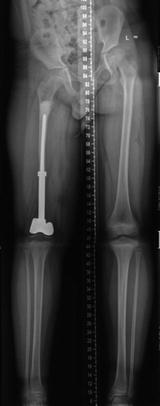

Fig. 11.8
Eight-year-old boy with a distal femur osteosarcoma that has been resected and the defect was constructed with a semi-articular knee endoprosthesis. A leg-length discrepancy of 5 cm developed at 4 years after reconstruction
Differences in length between upper extremities are not often a problem, but leg length discrepancies can cause significant functional deficits. The distal femur and proximal tibia are the most common sites for primary malignant bone tumors, and the epiphyses of the distal femur and proximal tibia contribute approximately 35 % and 30 %, respectively, to growth of the lower extremity [36]. Also, many other factors contribute to the overall limb length discrepancy, including systemic chemotherapy, slowing of the preserved growth plate in the affected joint, muscle atrophy, muscle loss and overgrowth of the contralateral limb. Several options, including conservative method and surgical intervention, such as expandable prosthesis, epiphyseal preservation, contralateral epiphysiodesis or distraction osteogenesis, have been developed to solve the problem. In order to choose the proper elongation method, it is important to estimate the expected limb length inequality resulting from both segmental resection and loss of subsequent normal growth. Several methods have been developed to estimate final height at skeletal maturity, including Paley’s “multiplier” method [62, 63], Moseley graph [64] and Green-Anderson growth remaining charts [65].
Leg length discrepancies less than 2 cm in the lower extremities is well tolerated and normally has little-to-no functional or clinical significance. These discrepancies can be easily corrected by shoe lift. Discrepancies of 2–5 cm are associated with gait abnormalities [66] and can be corrected with a shoe lift or a contralateral epiphysiodesis. Inequality larger than 5 cm often requires surgical management (Table 11.4).
Leg length discrepancy (cm) | Treatment |
|---|---|
0–2 | No treatment required |
2–5 | Shoe lift and/or epiphysiodesis |
5–15 | Lengthening, expandable prosthesis, distraction osteogenesis |
>15 | Multiple procedures, rotationplasty, amputation |
Distraction osteogenesis is a surgical process used to lengthen the long bones. After corticotomy, the two bone ends are gradually moved apart during the distraction phase, allowing novo bone to form in the gap (Fig. 11.9). It is based on accelerating the functional remodeling and integration seen with fracture healing [68, 69]. Distraction is usually started 7–14 days after the osteotomy, with a rate of 1 mm/day [70], however the consolidation phase is usually twice as long as the distraction phase. Duration is estimated on the basis of 1 month/cm of increased length [71, 72]. The most common complications using this method are pin tract infection, pin break, fracture of regenerate, dislocation/subluxation of nearby joint, skin invagination, bone resorption and loss of alignment. Tsuchiya et al. [73] reported a complication rate of 52.6 %. Physeal distraction procedures have the advantages of more rapid ossification, earlier weight bearing, and fewer complications than do other lengthening techniques but should be used only in children under 12. Cãnadell et al. used distraction osteogenesis to expand the tumor-free margin and to preserve the epiphysis by physeal distraction.