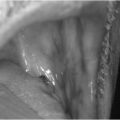
The filament is pressed against the plantar aspects of the first and fifth toes, the first, third and fifth metatarsal heads and the plantar surface of the heel (Fig. 40.1a). The monofilament buckles at a given force of 10g (Fig. 40.1b). The monofilament should not be applied at any site until callus has been removed.
Vibration sensation is assessed using a 128Hz tuning fork. A neurothesiometer, if available, can also be used to detect sensory neuropathy. It is a device that delivers a vibratory stimulus to the foot that increases as the voltage is raised.
Peripheral arterial disease
Peripheral arterial disease should be suspected in patients with intermittent claudication, cool temperature, absence of hair and presence of foot ulcers.
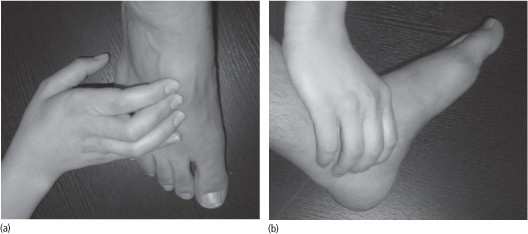
An absence of pedal pulses (Fig. 40.2) and a prolongation of venous filling should prompt further investigation and referral to the vascular surgical team. To measure the venous filling time, a prominent pedal vein is identified with the patient in a supine position. The leg is then elevated to 45° for 1 minute to collapse the vein. The patient then sits up and hangs the leg over the examination table. If more than 20 seconds elapse before the vein bulges above the skin, important arterial disease is likely to be present.
The ankle-brachial pressure index (ABPI) is calculated by measuring the systolic blood pressure (using a small hand-held Doppler) in the brachial, posterior tibial and dorsalis pedis arteries. The highest of four measurements in the ankles and feet is divided by the highest of the brachial measurements. The normal ABPI is 1.0–1.3. An ABPI of 0.90 or less is diagnostic of peripheral arterial disease. An ABPI over 1.30 suggests the presence of calcified vessels, which is common in diabetes.
Figure 40.1 (a) Sites for 10g monofilament testing. (b) 10g monofilament testing.
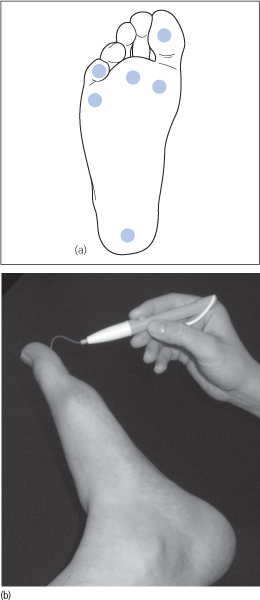
Figure 40.2 (a) Palpation of the dorsalis pedis pulse. (b) Palpation of the posterior tibial pulse.
Infected ulcers
Infections usually begin around cracks in the skin of the foot or around the toe nail bed (paronychia), or arise from ulcers. Diabetic foot infections are diagnosed clinically on the basis of:
- at least two of the following: erythema, warmth, swelling, tenderness
- pus coming out of an ulcer site or a nearby sinus tract.
Patients with cellulitis who have sensory neuropathy often do not experience pain. In necrotizing infections, purple/black discoloration of the skin, cutaneous bullae or soft tissue gas may occur.
Severity of ulcer infection
Diabetic foot infections may be mild, moderate or severe:
- Mild infections (involvement of the skin or superficial subcutaneous tissues): two or more markers of inflammation (erythema, warmth, swelling, tenderness), purulence and cellulitis extending for less than 2 cm around the ulcer.
- Moderate infections (more extensive infection or involvement of deeper tissues): cellulitis extending > 2 cm around an ulcer, lymphangitic streaking, deep tissue abscess, gangrene (necrosis) or involvement of muscle, tendon, joint or bone.
- Severe infections (signs of systemic toxicity or metabolic instability): fever, chills, tachycardia, tachypnoea, hypotension, confusion, vomiting and blood tests showing severe hyperglycaemia, leukocytosis, metabolic acidosis and raised urea and creatinine (see ‘Investigations’ below).
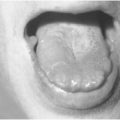
Figure 40.3 Diabetic foot with dry gangrene (caused by ischaemia), loss of hair and nail dystrophy.
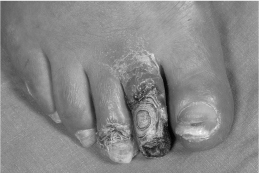
Gangrene (necrosis) is caused by ischaemia and is classified as wet or dry. In wet gangrene, ischaemia is caused by septic vasculitis associated with soft tissue infection. The tissues are black, brown or grey, moist and often malodorous. In dry gangrene, ischaemia is caused by peripheral arterial disease. The tissues are black, hard and mummified (Fig. 40.3). There is a clean demarcation line between necrosis and viable tissue.
Up to two-thirds of patients with diabetic foot ulcers may have osteomyelitis. The following factors increase the likelihood of osteomyelitis in patients with diabetic foot ulcers:
- visible bone or the ability to probe to bone (using a sterile, blunt, stainless steel probe)
- ulcer size >2 cm × 2cm
- ulcer depth >3 mm
- ulcer duration longer than 1–2 weeks
- erythrocyte sedimentation rate (ESR) >70 mm per hour (see ‘Investigations’ below).
Investigations
Blood tests
Blood tests should include full blood count, urea and electrolytes, ESR, C-reactive protein (CRP), blood glucose and glycated haemoglobin (HbA1c).
Ulcer swabs and bone biopsy
Diabetic foot ulcers should be swabbed for microscopy, culture and sensitivity. Organisms isolated from superficial swabs may not reflect the organisms responsible for deeper infections. In the setting of deep tissue infections or osteomyelitis, cultures of deep tissue should be obtained at the time of debridement.
The correlation between superficial swab culture and bone biopsy culture is poor (up to 20%). Bone biopsy allows a histopathological diagnosis as well as microbiological culture and sensitivity. However, it is not always possible or practical to perform a bone biopsy as the incision made for a biopsy may not heal in those with peripheral arterial disease. Therefore patients may be treated empirically for the expected pathogens.
The microbiology of diabetic foot ulcers varies depending on the extent of involvement:
- Superficial ulcers: aerobic Gram-positive cocci including Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pyogenes and coagulase-negative staphylococci. Methicillin-resistant Staphylococcus aureus should be presumed and treated empirically.
- Deep, chronic ulcers: Gram-negative bacilli such as Pseudomonas aeruginosa and Proteus mirabilis, in addition to the above pathogens.
- Ulcers with extensive local inflammation and signs of systemic toxicity should be presumed to have anaerobic organisms as well as the above pathogens.
In patients with chronic ulcers or those previously treated with antibiotics, infections are usually polymicrobial. When multiple organisms grow from a culture, it is difficult to determine which ones are true pathogens.
Imaging
In patients with diabetic foot infections, a foot radiograph must be performed to look for possible osteomyelitis.
If the foot radiograph is normal, the patient is treated for soft tissue infection for 2 weeks and the foot X-ray is repeated in 2–4 weeks. If the repeat radiograph remains normal, osteomyelitis is unlikely.
If either the initial or follow-up foot radiograph is characteristic of osteomyelitis, the person is treated for osteomyelitis after obtaining appropriate specimens for culture. Those with one or more of the risk factors for osteomyelitis (see above) whose radiographs are indeterminate for osteomyelitis should have a magnetic resonance imaging (MRI) scan (see below).
Radiographs are useful for demonstrating features of chronic osteomyelitis such as cortical erosion, periosteal reaction, mixed bony lucency, sclerosis and sequestra. Radiographs are of limited sensitivity and specificity in the detection of acute osteomyelitis. Osteolysis and periosteal new bone formation may not be evident until 2 weeks after onset of infection. However, MRI scanning demonstrates abnormal marrow oedema as early as 3–5 days after the onset of infection.
MRI identifies bone marrow oedema, soft tissue inflammation and cortical destruction. It cannot reliably differentiate between marrow oedema caused by osteomyelitis and that due to neuropathic (Charcot) arthropathy. MRI may also over-estimate the extent of infection, since marrow oedema caused by osteomyelitis and surrounding reactive oedema cannot be distinguished. Furthermore, bone marrow changes may persist for weeks to months after osteomyelitis begins to respond to treatment. Gadolinium contrast-enhanced MRI is useful for demonstrating sinus tracts, fistulas and abscesses.
The three-phase bone scan uses a radionuclide tracer that accumulates in areas of increased osteoblast activity (e.g. 99m technetium bound to a phosphorus-containing compound). Scans are performed immediately after tracer injection (blood flow phase), 15 minutes after injection (blood pool phase) and 4 hours after injection (osseous phase).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree