Introduction
Organisms undergo growth and development. They give birth to new individuals and multiply. They respond to internal and external stimuli to adapt to their environment and sustain life. They metabolize substances captured from outside of the body and excrete unnecessary ones. They continually use the energy generated by this metabolism to carry out life processes. Each cell and tissue is connected to each other through a network to perform these processes. The organism eventually undergoes senescence and death.
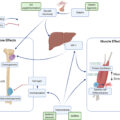
Bone and muscle are the two largest tissues in the musculoskeletal system. The main function of musculoskeletal system is locomotion, in which the mechanical interaction between bones and muscles work together. Both bone and muscle tissues act as a system of pulleys and levers whereby bones provide an attachment and muscles provide a load. There is a very close relationship between bones and muscles from embryogenesis to growth and development to aging. Bone and muscle develops synchronously during embryogenesis, which is regulated by multiple signaling pathways and stimulatory phenomena. Aging results in progressive bones loss known as osteopenia and osteoporosis and in progressive muscle loss known as sarcopenia. A healthy musculoskeletal system is essential for healthy people to regularly maintain physical activity, although aging results in decreases in physical performance and in nonmusculoskeletal systems. In the last two decades, the multiple findings have expanded our knowledge regarding the molecular and biomechanical interaction between bones and muscles.
In this chapter, we will focus on the structure and function of muscle and bone, changes in both tissues associated with growth and senescence, biochemical networks of muscle and bone tissue (cross-talk), and cytokines secreted by muscle and bone tissue that regulate muscle and bone health.
Structure and function of bones
Human bones undergo continuous renewal throughout life ( Fig. 1 ). This phenomenon is called modeling. The functions of bones include shaping the human body (body support), protecting the brain and internal organs (organ protection), maintaining static stability and performing dynamic activity together with muscles (body movement), storing calcium (99% of the calcium in the body), phosphorus (85% of the phosphorus in the body), and other minerals (mineral storage), and producing blood cells (blood cell production).
Bones are formed by calcification of the bone matrix secreted by osteoblasts, and there are two types of formation: intermembranous ossification and endochondral ossification. In both cases, undifferentiated mesenchymal cells differentiate into osteoblast progenitor cells and receive commitment to osteoblastic cells. Mature osteoblasts become osteocytes and are buried in the substrate secreted by the osteoblasts themselves, calcifying the bone matrix. These cells need to undergo the state of the germ layer from which they were originally derived, that is, the ectoderm, mesoderm, and endoderm, and then differentiate into specific cells of each tissue. Bones are classified according to their shape, and each bone is connected to each other by ligaments to form a skeleton that serves as a support for the body. Blood vessels and nerves in bone tissue are distributed in various parts of bone and are involved in the control of various functions. The bones also constitute the locomotorium by attaching tendons that connect the muscles. Bone tissue consists of a tight, dense cortical bone on the outside and a cancellous bone on the inside, with bone marrow filling the space between the trabeculae within the surface bone. The hematopoietic red bone marrow in childhood is present in bones throughout the body. With aging, the hematopoietic function of the long bones of the extremities is lost and replaced by fatty yellow bone marrow. However, in the bones of the trunk near the central axis of the body, such as the sternum and rib vertebrae, red bone marrow is present even in old age and is responsible for hematopoiesis.
During growth, the growing cartilage plates at both ends of the bone contribute to the longitudinal growth of long bones, but the cartilage endplates close in adulthood. The ends of the bone are covered with hyaline cartilage, which has no blood vessels or nerves, and constitutes a joint together with the synovium, which is rich in ligaments and vascular network. The outer surface of the bone other than the articular surface is covered with the periosteum, which is a strong fibrous connective tissue existing outside the dense bone. The outer side of the periosteum, which is rich in thick type I collagen fibers, provides tendon and ligament attachments, while the inner side, which is rich in cell fibers and rich in blood vessels, is involved in bone formation and maintenance.
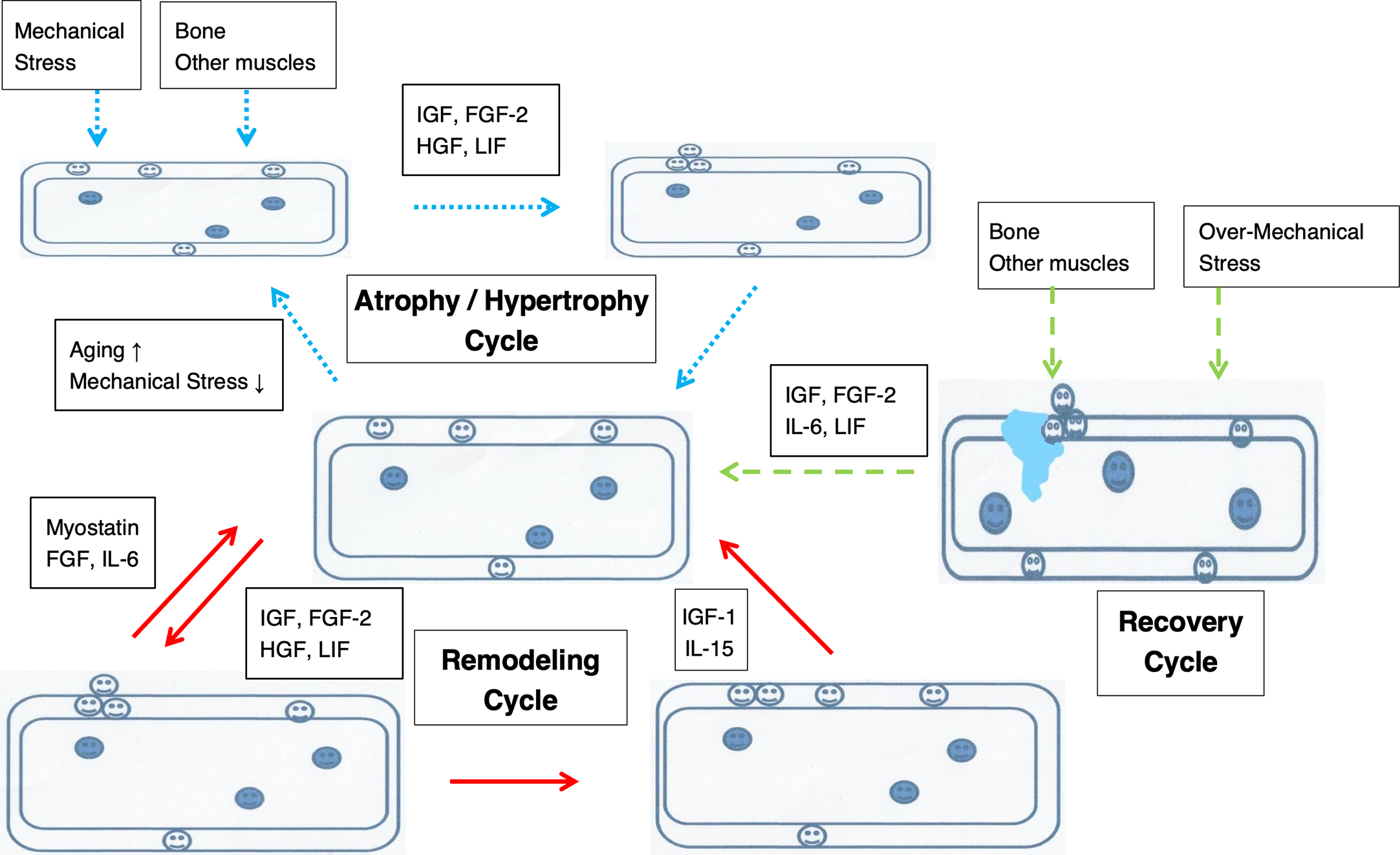
Bones change with age, and aging affects the quality and quantity of the bone matrix. Remodeling involves removal of old bone tissue and replacement with new bone tissue after bone shaping is complete. Remodeling affects bone strength whereby osteoclasts resorb bone, preosteoblasts that migrate and colonize, and osteoblasts and osteocytes that form bone. In addition to coupling factors that maintain communication between osteoclasts and osteoblasts, this remodeling required for maintaining bone strength has also been suggested to occur outside the bones due to mechanical stresses such as locomotion and the nervous and immune systems. Bone strength is dictated by hydroxyapatite, a crystal of calcium phosphate, as the main component of bones, and bone mineral density is dictated by the bone matrix, with type I collagen (collagen fiber) as the main component (for flexibility and binding of collagen molecules) . This chapter introduces the aforementioned coupling factors and the effects of aging; the external effects of locomotion are introduced in later chapters ( Fig. 2 ).

Differentiation and function of osteoclasts
Osteoclasts are multinucleated cells that control the resorption of mineralization and are modulated by osteocytes and osteoblasts that are involved in bone formation. Osteoclast activity is regulated by vitamin D, parathyroid hormone, calcitonin, and leptin. Osteoclast progenitor cells differentiating from the mononuclear phagocyte system possess the receptor activator of nuclear factor-κΒ (RANK) receptor, which binds RANK ligand secreted by osteoblasts. Osteoclasts maintain serum calcium homeostasis and bone resorption by promoting and suppressing bone resorption. In addition to the release and activation of the transforming growth factor-β (TGF-β) family, bone marrow mesenchymal stem cell recruitment and osteoblast differentiation promote bone regeneration. Leptin suppresses the proliferation of osteoblasts via the sympathetic nervous system and suppresses bone formation. This promotes the secretion of nuclear factor-κΒ ligand (RANKL) from osteoblasts and promotes bone resorption of osteoclasts. Osteoclast differentiation is regulated by RANK and M-CSF (macrophage colony-stimulating factor 1). Osteoclast progenitor cells express RANK receptors and M-CSF receptors, which recognize RANKL and M-CSF produced by osteoblasts, leading to differentiation and bone resorption. A decrease in blood calcium stimulates the secretion of parathyroid hormone (PTH) and promotes bone resorption by osteoclasts. In contrast, an increase in blood calcium promotes the secretion of calcitonin and suppresses bone resorption by osteoclasts. Regulation by cytokines and vitamins is explained below with effects of aging on bones and age-related osteoporosis.
Differentiation and function of osteoblasts
Osteoblasts derived from differentiation of mesenchymal stem cells activate intracellular factors via diverse signaling pathways and induce or suppress the expression of various mRNAs. Furthermore, the differentiation of bone cells is modulated by various transcription factors and humoral factors. The regulation of transcription involves Runx2 (Runt-related transcription factor-2) which belongs to the Runx family and Osterix which is a transcription factor induced by BMP2 (bone morphogenetic protein-2). Runx2 differentiates pluripotent undifferentiated mesenchymal cells into preosteoblasts, and Runx2 and Osterix are essential for subsequent mature osteoblast differentiation. Runx2 and Ostrix are also involved in chondrocyte proliferation and hypertrophy. The transcription factors Runx2 and Osterix are induced by type I collagen, noncollagen proteins such as osteocalcin and osteopontin, and cytokines that also modulate osteoblast differentiation and activation. Humoral factors, including the TGF-β family, regulate the migration and differentiation of bone-forming mesenchymal stem cells, which are involved in bone formation, into bone-resorption sites. Wnt, which promotes bone formation, and sclerostin, which suppresses bone formation, are involved in the regulation by humoral factors. Wnt binds to Wnt co-receptor LRP5, suppresses GSK-β (glycogen synthase kinase-β), and inhibits the degradation of β-catenin. The activated β-catenin translocates to the nucleus and promotes differentiation from pluripotent undifferentiated mesenchymal cells into osteoblasts. Sclerostin suppresses the action of Wnt, suppresses the differentiation and calcification of osteoblasts, and induces apoptosis. Osteocalcin, a hormone that acts on β cells of the pancreas to increase insulin secretory capacity, suppresses bone formation. Recent studies have reported that leptin, which is secreted by adipocytes with a contact-suppressing action, suppresses osteoblast proliferation and bone formation via sympathetic nerves. Thus, various transcription and humoral factors are involved in promotion and suppression of osteoblasts. Most osteoblasts die by apoptosis, but some are buried in the bone matrix produced by themselves and differentiate into mature osteocytes in the process of mineralization. Mechanical stimulation suppresses the action of sclerostin and promotes osteoblast differentiation.
Differentiation and function of osteocytes
Osteocytes are buried in the calcified osteoid as mature osteoblasts are incorporated into the bone matrix. Osteocytes produced by the differentiation of osteoblasts have projections that form a cellular network. Mechanical stress applied to the bones stimulates osteocytes to transmit a signal among themselves. Via the osteocyte projections, these signals modulate bone metabolism and the functions of both osteoclasts and osteoblasts on the bone surface. Furthermore, since most phosphorus is stored in bones, bone cells modulate phosphorus metabolism. Phosphorus in the blood is absorbed from the intestinal tract, and excreted and reabsorbed from the kidneys to control the phosphorus level in blood. Therefore, when blood phosphorus concentration increases, bone cells suppress resorption in the kidneys.
Thus, bone remodeling causes bone formation, and in a healthy adult, bone resorption and bone formation are in equilibrium to maintain bone strength and fragility.
Effects of aging on bones and age-related osteoporosis
The progressively older adult population in developed countries is increasing at risk for osteoporosis, which constitutes a major public health threat. With aging, bone resorption exceeds bone formation, and bone strength decreases due to a decrease in bone mass and deterioration of bone quality. This is called age-related osteoporosis. This chapter describes the morphological changes in age-related osteoporosis, endocrine and hormonal effects, and vitamin D effects.
Bone strength can be evaluated by changes in the bone mass of the cancellous (trabecular or spongy bones) and cortical bones (compact or substantia bones). Although there are various methods to measure bone strength, the current standard methods for bone density measurement include quantitative computed tomography and dual energy X-ray absorptiometry. In females, the age at puberty between 10.5 and 12.5 years overlaps with peak height development due to the burst in bone growth. Similarly, in males, the age at puberty (between 13 and 14 years) coincides with the peak in height development. At this peak, 26% of adult bone mass is acquired . Subsequently, the bone mineral density reaches the average value for young adults at 29 years of ages in the proximal femur and the lumbar spine. It is almost constant until 40 years old. Recent study presented reductions in cortical and tubercular in hydroxyapatite. At the distal radius of postmenopausal females, the decrease in hydroxyapatite at aged 50–64 years and at older than 80 years was −17.1 mg and −16.3 mg, respectively. However, the significant decrease in hydroxyapatite between aged 65 and 79 years was −73.1 mg, of which cortical bone was −50.2 mg and trabecular bone was −22.9 mg. The bone mass in human body composes 80% of trabecular and 20% of cortical compartment. Therefore, the cortical compartment, which is originally small volume in the bone compartment, is significantly reduced with aging. This quality of bone, is deteriorated, may result in the developing osteoporosis and stress fracture. .
In age-related osteoporosis, structural bone quality (bone microstructure) breaks down due to increased bone resorption and deterioration of material quality due to increased oxidative stress associated with menopause and aging . It has been reported that this oxidative stress is further exacerbated by lifestyle-related diseases . Further, advanced glycation end products (AGEs), which accumulate with age, affect bone fragility .
Sclerostin and Wnt family
Sclerostin, secreted by osteocytes, acts to suppress bone formation. Sclerostin, which is a product of the SOST gene, binds directly to the Wnt co-receptors LRP4 and LRP5. This inhibition induces osteoblast differentiation, proliferation, and activity resulting in bone formation. The Wnt/β-catenin signaling pathway promotes bone formation and osteocyte transmission of mechanical loading by binding Wnt/β-catenin to its receptors Frizzled and co-receptors LRP4 and LRP5. The pathway is triggered by cross-talk with the prostaglandin pathway in response to loading, thereby decreasing negative regulators of bone formation such as sclerostin. Sclerostin suppresses the differentiation and calcification of osteoblasts responsible for bone formation and induces apoptosis of bone cells. Mechanical stress and parathyroid hormone (PTH) suppress TGF-β-mediated SOST expression, suppress sclerostin production, and promote bone formation. Vitamin D, also, increases bone formation in a manner inversely related to sclerostin. The marker of osteocyte mechano-sensing is sclerostin, which suppresses stimulation of bone formation by differentiated osteocytes.
Estrogen
Estrogen enhances the function of osteoblasts and suppresses the function of osteoclasts. It also increases vitamin D synthesis in the kidneys. Therefore, when estrogen secretion decreases due to menopause, osteoblastic bone formation is reduced and osteoclast bone resorption is enhanced, resulting in osteoporosis.
Vitamin D
When calcium metabolism is normal, active vitamin D promotes calcium absorption in the small intestine and reabsorption of calcium in the kidneys to maintain the calcium concentration in the blood, resulting in bone formation. Active vitamin D promotes the deposition of calcium and contributes to bone formation. On the other hand, when the calcium concentration in the blood decreases, the synthesis of active vitamin D in the kidneys increases, and in collaboration with PTH, calcium is removed from the bones and the homeostasis of the calcium concentration in the blood is maintained. A recent study demonstrated that vitamin D increases bone formation in an animal model for osteoporosis .
Vitamin D is abundant in blood and control two different receptors for sphingosine-1-phosphate (S1P), a lysophospholipid mediator enriched in blood. These receptors play a vital role in regulating the migration and positioning the osteoclast precursors on the bone surface . One receptor is S1PR1 perform positive chemotaxis to S1P and is involved in the migration of osteoclast precursor cells from the bone surface to blood vessels. Another receptor is S1PR2 play negative chemotaxis to S1P. Osteoclast precursors control the activity and inhibition of bone resorption via these two receptors. Therefore, the active vitamin D promotes bone formation and control bone remodeling. .
Structure and function of muscle
Muscles are involved in various physical activities. Different shapes of muscles have different functuion and role. The function of muscle includes body movement, posture retention, breathing, oxygen and energy consumption, heat production, organ and blood vessel construction, heart contraction and relaxation, glycogen storage, external shock buffering, and blood circulation support (milking action). Muscles are classified into three types according to their function: skeletal muscles, smooth muscles, and myocardium. Skeletal muscles and myocardium have a striated structure whereas smooth muscles and myocardium are involuntary muscles. Thus, the myocardium is a muscle with structural features of skeletal muscle and neurological features of smooth muscle. This section introduces the physiological characteristics and functions of skeletal muscles and the endocrine organs of skeletal muscles.
Skeletal muscles contract spontaneously and account for approximately 40% of body weight. Skeletal muscles are generally attached to the bone by tendons, which are connective tissues that attach the muscle to the periosteum of the bone. The muscle fiber structure of skeletal muscles has a parallel or pennate arrangement. Muscle spindles have bundles that are organized parallel to the semimajor axis of the muscle. Muscle spindles are also greatly shortened because the fasciculation lines up directly with the tendons. The lesser the number of total bundles attached to the tendon, the lesser force required to contract the muscle spindle. Therefore, spindle-shaped muscles can generate a large force quickly. For this reason, they are involved in the function of elbow and shoulder joints, which move quickly and with great mobility. Pennate muscles have a bundle associated with the tendon and can span the length of the entire muscle. The long tendons of skeletal muscles may extend to some distance between the origin and insertion of the muscle. Due to its pennate shape, many bundles are attached to a single tendon. Examples of pennate muscles are quadriceps femoris and calf muscles, which can generate as well as withstand large outside forces. Skeletal muscle is composed of skeletal muscle, nervous, connective, and adipose tissues.
Skeletal muscles are surrounded by several connective tissue layers that support the muscle during contraction. Skeletal muscles are composed of three layers of connective tissue: epimysium, perimysium, and endomysium. In addition, there are two types of skeletal muscle cells: slow-twitch (type I) and fast-twitch fibers (FT or type II), depending on their contractile characteristics. The composition ratio of these fibers are different in each muscle and do not have the same functional capacity. These muscle fiber types are controlled by myosin heavy chains and molecules, blood supply, motor neurons, and mitochondria for exertion and endurance. Type I muscle fibers have high oxidase activity due to their volume density and large number of mitochondria. They are reddish due to their high myoglobin content and capillary density. Therefore, type I muscle fibers contract slowly, have a large blood supply, and are more resistant to fatigue. Type II muscle fibers exert great force and contract at a high rate because of high sarcoplasmic reticulum content. Their glycolytic enzyme activity is high due to the large amount of glycogen stored in cells. The composition of type I and II muscle fibers is affected by genetics regardless of gender. Type II muscle fibers have high contractile resistance and oxidase activity, and can be divided into subtypes: type IIa (FT oxidative glycolytic fiber) and low type IIb (FT glycolytic fiber).
For skeletal muscle, isometric contraction (static contraction) is involved in posture maintenance, isotonic contraction (concentric and eccentric contraction) is involved in physical activity accompanied by muscle shortening and stretching, and isokinetic contraction is involved in exerting tension with constant velocity. Isometric contraction occurs when a muscle that develops tension is unable to move the load and does not change length. When a muscle maintains a constant length, it produces increasing tension. Muscle strength is measured when isometric contraction action is performed with maximum effort; this is referred to as maximum muscle strength. The effort exerted by the muscle is insufficient to move when the load is too heavy or when dealing muscles contract in opposition to each other, thus preventing movement. This is called engaged isometric contraction. The effort during the contracted isometric contraction is higher the isometric contraction by 10%.
There are two types of isotonic contraction: concentric and eccentric contraction. Concentric contraction means that the muscle develops sufficient tension to overcome resistance and shortens. Eccentric contraction means that the muscle produces tension as it resists lengthening. Eccentric contraction may occur when muscle is used to oppose a movement, but not to stop it. When an equal load is applied, the tension created in a muscle during an eccentric contraction is higher than that created during a concentric contraction. Eccentric contraction can induce delayed-onset muscle soreness (DOMS) which leads to an increase in inflammation, pain, and muscle damage along with a decrease in strength and range of motion. When DOMS occurs, interleukin-6 (IL-6), cortisol, and creatine kinase significantly increase for the few days after the first eccentric contraction training. The combination of eccentric and concentric contraction is referred to as a stretch-shortening cycle (counter-movement). Putting a muscle under stretch in the eccentric contraction phase enables the muscle to store potential (elastic) energy.
Thus, a mixture of isotonic and eccentric contractions, and complex elongation-shortening cycles are used in everyday life. The effects on the muscle tissue are different since the muscles are loaded differently. Subsequently, the quality and quantity of muscle damage and various secreted substances differ. Eccentric contraction affects the secretion of a large number of humoral factors for muscle regeneration such as IL-6, IL-15, and cortisol.
Growth and development of muscles and effects of aging on muscles
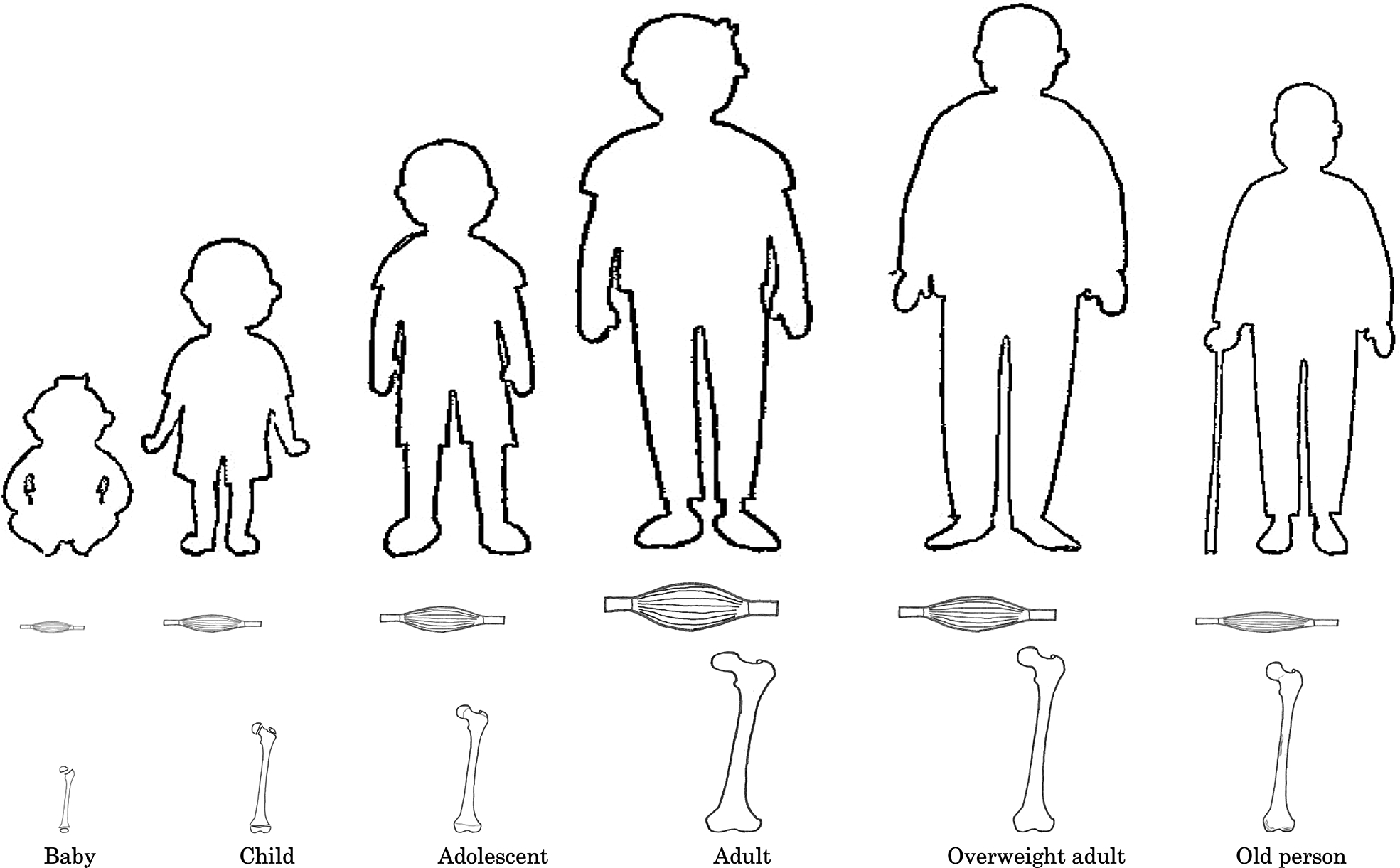
Skeletal muscle cells, similar to central nerve cells, are mitotic cells that do not undergo cell division or regeneration after birth. The increase in skeletal muscle weight from infancy to adolescence is due to cell hypertrophy and vertical growth due to increased cell and sarcomere numbers.
A positive correlation has been found between the growth and development of muscle fibers between the ages of 7 and 18 and voluntary muscle strength. Voluntary muscle exertion in humans is dictated by the level of excitement of the cerebral cortex, rate of motor nerve participation, and cross-section of a unit muscle. Muscle fiber development during this period involves hypertrophy of myofibrils and extension of muscle length, but in children up to 10 years old, muscle strength per unit area tends to be low. This is thought to be due to the underdeveloped nervous system function. In addition, gender differences are observed after the age of 13; in girls, there is a difference in muscle cross-sectional area and strength due to an increase in blood estrogen concentration, which suppresses long bone growth and white muscle fiber hypertrophy .
Age-related loss of muscle strength and mass increases the likelihood of bed-rest or long-term care due to decreased activity in the elderly, injuries due to falls, and the onset of sarcopenia. The rate of decrease in muscle mass, number of muscle fibers, and muscle strength with aging is consistent. The loss of muscle mass results from changes in the balance between protein synthesis and protein degradation. The decrease in secreted IGF-1 and physical activity, bed rest, lack of nutrition and chronic diseases (including noncommunicable diseases) have been related to enhanced protein degradation and depletion of muscle mass. The decrease in hormones also appears to impact the pathogenesis of muscle loss. The reduced levels of estrogen and testosterone may indirectly affect muscle mass due to their inhibitory effects on the regulation of IL-1 and IL-6, which are pro-inflammatory and pro-catabolic cytokine. These changes are related to maximum muscle strength (isometric muscle strength), which depends on muscle mass and the maximum tension generated by motor units during concentric contraction; thus, muscle power is not significantly affected by aging. Various physiological mechanisms are involved in the power reduction of lower limb muscle strength in elderly people with healthy muscle strength per unit cross-section of muscle and mobility-limited older adults . Thus, age-related muscle weakness is due to decreased neuromuscular facilitation rather than decreased muscle mass in FT muscle fibers. The single surviving muscle fasciculation function acts as a compensatory action to maintain the required strength and function in the event of age-related loss of major muscle groups throughout the body ( Fig. 3 ).