Autologous stem cell transplant (ASCT) remains an integral part of the treatment strategy for many myeloma patients. The role of allogeneic stem cell transplant continues to be defined. There is increasing evidence that posttransplant maintenance therapy can significantly improve outcomes. It is predicted that with more routine use of cytogenetic and gene expression profiling in the future, we will be better able to identify those subgroups of patients who are expected to benefit most from early versus late versus no ASCT and those who will benefit from allogeneic stem cell transplant.
Key points
- •
Patients eligible for high-dose therapy typically undergo induction therapy followed by upfront autologous stem cell transplant. Whether transplant can be delayed until time of first relapse with equivalent long-term outcomes is currently under study.
- •
Single-agent high-dose melphalan remains the standard of care for the high-dose regimen for autologous stem cell transplant.
- •
Reduced-intensity conditioning regimens for allogeneic transplant have improved transplant-related mortality rates, but disease relapse, graft-versus-host disease, and treatment-related mortality remain significant problems.
- •
Salvage autologous stem cell transplant is feasible and seems to be most effective for patients who have relapse at least 18 months or later from their initial transplant.
- •
The therapeutic landscape for myeloma is evolving rapidly, impacting both pre- and posttransplant treatment strategies.
Introduction
Induction therapy followed by consolidation with high-dose melphalan and autologous stem cell transplant (ASCT) has been considered the standard of care for transplant-eligible myeloma patients for several decades. The role of allogeneic stem cell transplant (AlloSCT) has been less clear, as both myeloablative and reduced-intensity approaches have been fraught with transplant-related mortality, graft versus host disease (GvHD), and disease relapse. In today’s era of novel agents, which include the immunomodulatory agents (thalidomide, lenalidomide, pomalidomide) and proteasome inhibitors (bortezomib and carfilzomib), several unanswered questions remain regarding the role of transplant. These unresolved issues include the timing of initial transplant (upfront after induction therapy vs delayed until time of first relapse or later), the incorporation of novel agents into the high-dose regimen, the optimal maintenance regimen after transplant, the role of novel agents versus salvage (second) transplant after relapse from the first transplant, and the role of AlloSCT. In this article we provide an overview of transplantation for myeloma and discuss the areas that remain under active investigation.
Introduction
Induction therapy followed by consolidation with high-dose melphalan and autologous stem cell transplant (ASCT) has been considered the standard of care for transplant-eligible myeloma patients for several decades. The role of allogeneic stem cell transplant (AlloSCT) has been less clear, as both myeloablative and reduced-intensity approaches have been fraught with transplant-related mortality, graft versus host disease (GvHD), and disease relapse. In today’s era of novel agents, which include the immunomodulatory agents (thalidomide, lenalidomide, pomalidomide) and proteasome inhibitors (bortezomib and carfilzomib), several unanswered questions remain regarding the role of transplant. These unresolved issues include the timing of initial transplant (upfront after induction therapy vs delayed until time of first relapse or later), the incorporation of novel agents into the high-dose regimen, the optimal maintenance regimen after transplant, the role of novel agents versus salvage (second) transplant after relapse from the first transplant, and the role of AlloSCT. In this article we provide an overview of transplantation for myeloma and discuss the areas that remain under active investigation.
Patient evaluation overview
As denoted in Table 1 , the standard pretransplant evaluation includes assessment of organ function, infectious disease status, psychosocial status, and myeloma restaging. Human leukocyte antigen (HLA) typing is performed for patients being considered for AlloSCT and possibly for younger patients being considered for autologous stem cell transplant who could potentially be offered AlloSCT in the future.
| Test | Notes |
|---|---|
| Renal function | Creatinine clearance <50 mL/min may result in a dose modification of the high-dose melphalan |
| Hepatic function | Direct bilirubin, alkaline phosphatase, AST/ALT <3× normal |
| Myeloma restaging | Quantitative immunoglobulins, serum immunofixation electrophoresis (IFE), serum protein electrophoresis, urine IFE, urine protein electrophoresis, serum free light chains, skeletal survey, bone marrow aspirate/biopsy with standard karyotyping and FISH panel of CD138-selected cells, flow cytometry |
| PET/CT scan | Considered for patients with plasmacytoma |
| Pulmonary function tests | DLCO or DLVA ≥50% predicted; DLCO to be corrected for hemoglobin and/or alveolar ventilation |
| Cardiac function: ECG and echocardiogram/MUGA | Left ventricular ejection fraction (LVEF) ≥50% or cardiology consult if LVEF <50% |
| Osteoporosis evaluation | DEXA, vitamin D |
| Infectious disease testing | CMV immunoglobulin G/immunoglobulin M, hepatitis B/C, HIV, HTLV, Treponemal pallidum |
| HLA typing | If AlloSCT to be considered |
| Psychosocial evaluation | Social work evaluation, patient and caregiver orientation, family meeting |
| Dental consult | To evaluate for osteonecrosis of the jaw or severe dental problems that could complicate bisphosphonate use |
| Physical therapy consult | Maintaining strength after transplant |
| Dietary consult | Consideration for nutritional supplementation such as total parenteral nutrition following transplant |
Indications for transplant
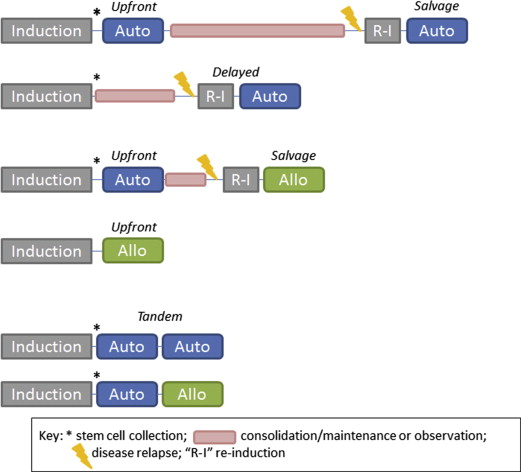
Traditionally, all patients younger than 65 years with adequate organ function and performance status and who have achieved disease control have been considered candidates for ASCT. Most transplant centers in the United States, however, will perform transplants on patients up to the age 75, and some centers have no age limit but instead rely on performance status and adequate organ function. Multiple studies have shown the feasibility and efficacy of performing ASCT in elderly patients. The role of AlloSCT in myeloma continues to be debated. Outside of a clinical trial, it is most often considered for younger patients with high-risk disease in either the upfront setting or after an early relapse after ASCT ( Box 1 , Fig. 1 ).
Autologous
- •
Upfront: patients ≤75 (or older?) years of age who have achieved best response after induction therapy
- •
Delayed: patients ≤75 (or older?) years of age who have relapsed after initial therapy and who have achieved disease control. Stem cells are usually collected after initial best response but may also be collected after treatment of first relapse
- •
Salvage: patients ≤75 (or older?) years of age who have relapsed after initial autologous transplant (preferably after a minimum of 18 months of disease control from the initial transplant) and who have again achieved disease control
Allogeneic
- •
Upfront: patients ≤40–50 years of age with high-risk disease features (young age; del 17p, t[4;14], t[14;16]; plasma cell leukemia; high lactate dehydrogenase level, and high-risk gene expression profile [GEP70 in USA, EMC 92 in Europe]) who have achieved best response after induction therapy
- •
Salvage: patients ≤65 years of age with high-risk disease (young age; del 17p, t[4;14], t[14;16]; plasma cell leukemia, high lactate dehydrogenase level, high-risk gene expression profile) features who have relapsed after initial autologous stem cell transplant
Autologous stem cell transplant
The most widely used high-dose regimen is single-agent melphalan at a dose of 200 mg/m 2 (MEL200). As shown in the text box below, MEL200 has been compared with lower doses of melphalan, melphalan in combination with total body irradiation (TBI), melphalan in combination with other agents, and non–melphalan-containing regimens. In aggregate, the toxicity profiles and disease-related outcomes have favored MEL200. It should be noted that there is variation among the studies with respect to timing of melphalan and stem cell infusion and whether melphalan is given over 1 day or 2 days. In general, melphalan is dose reduced to 140 mg/m 2 (MEL140) for patients with renal insufficiency (generally creatinine clearance <40) although there are limited data to support this practice. MEL140 is also considered for patients older than 70 years.
More recently, there has been interest in adding bortezomib to high-dose melphalan. While only small, nonrandomized studies have been conducted, these studies do demonstrate tolerability of this approach. The number of doses and timing of bortezomib administration with respect to melphalan administration and stem cell infusion has not yet been fully established ( Box 2 ).
- •
MEL200 versus MEL140 + TBI
- ○
MEL200 associated with faster hematologic recovery, fewer transfusions, shorter hospitalization, and less severe mucositis.
- ○
There was no difference in PFS and OS.
- ○
- •
MEL200 versus MEL100 (tandem transplants)
- ○
MEL200 associated with more severe thrombocytopenia, mucositis, and gastrointestinal adverse events.
- ○
MEL200 associated with a statistically significant improvement in median PFS.
- ○
- •
MEL200 (over 2 days) versus thiotepa/busulfan/cyclophosphamide (not randomized)
- ○
No differences in response rates, PFS, OS.
- ○
- •
MEL200 (over 2 days) versus MEL100 (over 2 days)/idarubicin/cyclophosphamide
- ○
Study terminated because of a treatment-related mortality rate of 20% in the investigational arm compared with 0% in the standard melphalan arm.
- ○
No differences in response rates, PFS, OS.
- ○
- •
MEL200 versus MEL60 (over 3 day)/thiotepa/etoposide (not randomized)
- ○
Combination arm associated with higher number of febrile days, more total parenteral nutrition.
- ○
Median PFS and OS higher in the combination arm.
- ○
- •
MEL200 (over 1 or 2 days) versus MEL140/busulfan (BUMEL) (not randomized)
- ○
First 225 patients received BUMEL, but interim analysis showed high incidence of veno-occlusive disease (8%). Remaining patients received MEL200.
- ○
No differences in response rates or OS but 5-year PFS better with BUMEL.
- ○
Allogeneic stem cell transplantation
The initial studies that were performed using AlloSCT with myeloablative regimens were associated with unacceptably high early mortality rates ranging from 16% to 50%. Thus, the field has moved away from myeloablative regimens and is instead focusing on reduced-intensity conditioning or nonmyeloablative approaches. Several phase II studies with reduced-intensity conditioning allografts have been performed. The conditioning regimens have primarily consisted of fludarabine plus melphalan with or without antithymocyte globulin (ATG) or TBI. Rates of chronic graft-versus-host disease (GVHD) and transplant-related mortality have ranged from 27% to 58% and 15% to 40%, respectively. There are data to support graft versus myeloma effect, as there is improved progression-free survival (PFS) and overall survival (OS) for patients with chronic GVHD as opposed to those without, and donor lymphocyte infusions are associated with antimyeloma activity.
Timing for transplantation
The possible scenarios for sequencing and timing of transplants are shown in Fig. 1 . Historically, ASCT has been performed in the upfront setting after induction therapy. In today’s era of active novel agents, which can induce deep responses, it is unknown whether ASCT can be delayed until the time of first relapse. A summary of recent and ongoing trials addressing this question is shown in Box 3 . In all of these trials, stem cells are collected after induction therapy.
- •
IFM/DFCI 2009 DETERMINATION (ongoing)
- ○
Lenalidomide/bortezomib/dexamethasone (RVD) × 3 induction followed by randomization to either (1) MEL200-ASCT with RVD × 2 consolidation and lenalidomide maintenance or (2) RVD × 5 and lenalidomide maintenance
- ○
- •
EMN02 (ongoing)
- ○
Bortezomib/cyclophosphamide/dexamethasone induction followed by randomization to either (1) bortezomib/melphalan/prednisone × 4 or (2) single or tandem MEL200-ASCT. Second randomization to either (1) lenalidomide maintenance or (2) RVD × 2 consolidation followed by lenalidomide maintenance
- ○
- •
MPR versus Mel200
- ○
Lenalidomide/dexamethasone × 4 induction followed by randomization to either (1) melphalan/prednisone/lenalidomide (MPR) × 6 or (2) tandem MEL200-ASCT. Second randomization to either (1) lenalidomide maintenance or (2) no maintenance.
- ○
Median PFS of 22.4 months (MPR) versus 43 months (MEL200) ( P <0.001) with no statistical difference in 4-year OS.
- ○
Median PFS and 3-year OS of 41.9 months and 88% (R maintenance) versus 21.6 months and 79.2% (no maintenance) ( P <.001 and P = .14).
- ○
- •
CRD versus MEL200
- ○
Lenalidomide/dexamethasone × 4 induction followed by randomization to either (1) cyclophosphamide/lenalidomide/dexamethasone (CRD) × 6 or (2) MEL200-ASCT. Second randomization to maintenance with either lenalidomide/prednisone (RP) or lenalidomide (R) maintenance.
- ○
With a median follow-up of 31 months, the median PFS was not reached in the MEL200-ASCT arm and was 28 months in the CRD arm. No difference in OS has been noted thus far. The 2-year PFS from starting maintenance was 73% for RP and 56% for R ( P = .03).
- ○
Tandem autologous versus single autologous transplantation
Once the benefit of ASCT was established in myeloma, several groups explored the approach of performing back-to-back, or tandem, ASCTs. The first randomized trial performed by Attal and colleagues found improved event-free survival (EFS) and OS with tandem transplant. However, subsequent studies did not uniformly show benefit of tandem transplant over a single transplant. A meta-analysis of 6 randomized clinical trials concluded that tandem transplant was associated with a statistically significant better response rate but at the cost of an increased transplant-related mortality rate. There was no difference in EFS or OS. Currently, most transplant centers in the United States do not routinely perform tandem autologous transplants outside of a clinical trial. Both the BMT CTN 0702 and EMN 02 trials are addressing the question of whether single or tandem ASCT is superior along with the role of consolidation.
Tandem autologous versus autologous-allogeneic transplantation
Several studies comparing upfront tandem ASCT with ASCT followed by AlloSCT have been performed and are summarized in Box 4 . Several of these studies used a biologic assignment approach in which patients who had an HLA-identical sibling donor received the autologous-allogeneic transplantation, whereas patients who did not have a sibling match underwent double ASCT. In aggregate, these studies do not show a significant benefit for tandem autologous-allogeneic transplant, and this approach is not routinely recommended outside of a clinical trial.
- •
IFM99-03/IFM99-04
- ○
No difference in EFS and trend toward improved OS in double auto arm
- ○
- •
PETHEMA
- ○
Higher complete response (CR) rates after autologous/allogeneic but no difference in EFS or OS
- ○
- •
Italian study
- ○
Higher CR rates and improved PFS and OS in allogeneic arm
- ○
- •
BMT CTN
- ○
No difference in PFS or OS
- ○
- •
HOVON-50/54
- ○
No difference in CR rate, PFS, or OS
- ○
- •
German DSMM
- ○
Higher CR rate in autologous/allogeneic but no difference in OS
- ○
- •
EBMT-NMAM200 (compared autologous/allogeneic with single or double ASCT)
- ○
Higher PFS, OS, and nonrelapse mortality rates in autologous/allogeneic
- ○
Posttransplantation monitoring
The standard day-100 evaluations include restaging studies assessing for monoclonal protein as well as a bone marrow aspirate and biopsy ( Table 2 ). There is increasing interest in the detection of minimal residual disease (MRD) as patients who achieve MRD-negative status seem to have improved outcomes compared with those who are MRD positive. Two major techniques under investigation are multiparameter flow cytometry and allele-specific oligonucleotide polymerase chain reaction (ASO PCR), which have sensitivities in the 10 −4 to 10 −5 range. Consensus guidelines regarding the use of multi-parameter flow cytometry will be forthcoming. The routine use of ASO-PCR has been limited by the requirement to make patient-specific primers.
| Test | Notes |
|---|---|
| Myeloma restaging | Quantitative immunoglobulins, serum and urine IFE, serum and urine protein electrophoresis, serum free light chains, skeletal survey, bone marrow aspirate/biopsy with standard karyotyping and FISH panel of CD138-selected cells, flow cytometry |
| PET/CT scan | Considered for patients with plasmacytoma |
| Osteoporosis evaluation | DEXA, vitamin D |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree