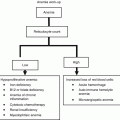
Adverse event
Novel agent
Action
Dose-reductions
Neutropenia
Lenalidomide, Bortezomib
G-CSF until neutrophil recovery in case of uncomplicated grade 4 or grade 2–3 complicated by fever or infection.
25–50 % drug reduction
Thrombocytopenia
Bortezomib, Lenalidomide
Platelet transfusion in case of occurrence of grade 4.
25–50 % drug reduction
Anaemia
Bortezomib, Lenalidomide
Erythropoietin or darbepoietin if hemoglobin level is ≤10 g/dL.
25–50 % drug reduction
Infection
All
Trimetoprin-cotrimoxazole for Pneumocystis carinii prophylaxis during high-dose dexamethasone. Acyclovir or valacyclovir for HVZ prophylaxis during bortezomib-containing therapy.
25–50 % drug reduction
Neurotoxicity
Bortezomib, Thalidomide
Neurological assessment before and during treatment. Immediate dose reduction is needed
Bortezomib: 25–50 % reduction for grade 1 with pain or grade 2 peripheral neuropathy; dose interruption until resolution to grade 1 or better with restart at 50 % dose reduction for grade 2 with pain or grade 3 peripheral neuropathy; treatment discontinuation for grade 4 peripheral neuropathy.
Thalidomide: 50 % reduction for grade 2 neuropathy; discontinuation for grade 3; resume Thalidomide at a decreased dose if neuropathy improves to grade 1.
Skin toxicity
Thalidomide, Lenalidomide
Steroids and antihistamines.
Interruption in case of grade 3–4.
50 % reduction in case of grade 2.
Gastrointestinal toxicity
All
Appropriate diet, laxatives, physical exercise, hydration, antidiarrheics.
Interruption in case of grade 3–4
50 % reduction in case of grade 2.
Thrombosis
Thalidomide, Lenalidomide
Aspirin 100–325 mg if no or one individual/myeloma thrombotic risk factor is present. LMWH or full dose warfarin if there are two or more individual/myeloma risk factors and in all patients with thalidomide-related risk factors.
Drug temporary interruption and full anticoagulation, then resume treatment
Renal toxicity
Lenalidomide
Correct precipitant factors (dehydration, hypercalcemia, hyperuricemia, urinary infections, and concomitant use of nephrotoxic drugs).
Reduce dose according to creatinine clearance:
If 30–60 mL/min: 10 mg/day;
If < 30 mL/min without dialysis needing: 15 mg every other day;
If < 30 mL/min with dialysis required: 5 mg/day after dialysis on dialysis day.
Young Patients
ASCT is considered the standard of care in younger patients. However, not all the studies that compared ASCT and conventional chemotherapy demonstrated the superiority of the former over the latter one [17–19]. Eventually, a meta-analysis of randomized studies including 2,411 patients treated with ASCT versus conventional chemotherapy, found that ASCT led to longer PFS, although no significant OS improvement was noted as compared with the conventional approach [20].
The choice of single versus double ASCT is still under debate and this issue has been addressed in various studies [11–24]. Despite conflicting results, particularly in terms of OS, all the studies reported a PFS advantage with tandem ASCT. A study confirmed the superiority of double vs a single ASCT after a long-term follow-up [25]. Because depth of response, particularly the achievement of CR, is a prognostic factor of improved survival [26–28], performing a second ASCT should be suggested in those patients who failed to achieve at least very good PR (VGPR) after the first ASCT [21, 22, 24, 28, 29].
Timing of transplantation is a very important question. In a pilot study, despite OS being equivalent with early or delayed ASCT, EFS and quality of life were improved in patients who underwent ASCT as upfront therapy [30]. A Spanish study showed that patients not responding to induction treatment benefited more from early ASCT [31].
Because of the widespread use of the novel agents as upfront therapy in newly diagnosed MM patients (NDMM), the role of early or delayed ASCT needs to be re-evaluated. Recently, a phase 3 study evaluated whether novel agent-containing regimens used as induction, consolidation and maintenance could delay ASCT to the time of first relapse [32]. This study confirmed the superiority of ASCT vs consolidation with lenalidomide in terms of both PFS and OS, showing that ASCT should not be delayed until time of relapse.
Upfront ASCT is typically preceded by a limited number of cycles of induction therapy, whose aim is to reduce tumour cell mass and infiltration before the collection of peripheral blood stem cells. The efficacy shown by novel agents in the relapse/refractory setting, led to the incorporation of such drugs in induction schemas for NDMM patients.
The combination vincristine-doxorubicin-dexamethasone (VAD) was the standard induction treatment for many years. Four to six cycles of VAD would lead to partial response (PR) rate of 52–63 %, with 3–13 % of CR rate [33]. Conventional induction followed by a single or tandem ASCT resulted in a CR rate of 30–40 %.
Novel agents in combination with established anti-myeloma drugs, such as dexamethasone, doxorubicin, or cyclophosphamide, have challenged the role of standard VAD, and new more effective induction regimens are now available.
Thalidomide-Containing Regimens
Various studies showed that the association of thalidomide plus dexamethasone (TD) as induction regimen before ASCT was superior in terms of response, event-free survival (EFS) and OS, if compared with traditional VAD regimen [34, 35]. As a result, TD has been one of the most commonly used induction regimens before ASCT for the past decade. The positive results obtained with TD provided the basis to evaluate the use of this combination with the addition of cytotoxic drugs, such as doxorubicin or cyclophosphamide, for the treatment of transplant-eligible patients. Both doxorubicin [36] and cyclophosphamide [37] plus TD, followed by double ASCT, led to a significantly higher VGPR/ CR rate and longer PFS compared to VAD induction. In the MRC Myeloma IX trial, in the intensive pathway, patients were randomized to receive TD plus cyclophosphamide (CTD) or cyclophosphamide-vincristine-doxorubicin-dexamethasone (CVAD) before transplantation [37]. After induction, CR rate was 13 % with CTD and 8 % with CVAD. Median PFS was 27 months for CTD and 25 months for CVAD, and OS was comparable in the two treatment arms as well (median not reached for CTD and median 63 months for CVAD). Because of its oral administration and the reduced incidence of infection (grade 3: 9 % vs 16 %) and cytopenia (grade 4: 2 % vs 9 %), CTD may be preferred. Peripheral neuropathy and deep vein thrombosis are some of the most important side effects of thalidomide therapy. Thromboprophylaxis, performed with aspirin in low-risk subjects, and warfarin or low molecular weight heparin in high-risk ones, is recommended in patients treated with thalidomide and high-dose dexamethasone or doxorubicin, and should be tailored according to the presence of individual risk factors for thromboembolic events. When thalidomide is used, peripheral neuropathy and deep vein thrombosis (DVT) may quite commonly occur, inducing a high drug-discontinuation rate.
Lenalidomide-Containing Regimen
Considering the good results obtained with TD combination, a phase 3 randomized trial assessed the role of lenalidomide in association with dexamethasone. Lenalidomide plus high-dose dexamethasone (RD) was compared with lenalidomide plus low-dose dexamethasone (Rd) as induction regimen both in myeloma patients eligible and not eligible for ASCT [38]. RD showed to be more effective than Rd with at least VGPR rate of 42 % vs 24 % respectively. However, the high dose regimen was associated with higher toxicity and early mortality rate, and did not result into longer PFS and OS. In a landmark analysis, the 3-year OS was about 92 % in patients who received ASCT after both RD and Rd.
The association of RD with bortezomib (VRD) showed to be effective and safe in a phase 1/2 study [39]. The maximum tolerated dose (MTD) was determined as bortezomib 1.3 mg/m2, lenalidomide 25 mg, and dexamethasone 20 mg. All of the patients (100 %) achieved at least a PR, including 30 % of CR. Twenty-eight out of sixty-six patients (42 %) proceeded to transplantation. The estimated 1.5-year PFS and OS for the combination treatment with/without transplantation were 75 and 97 %, respectively. Grade 3–4 hematologic toxicities included neutropenia (9 %), and thrombocytopenia (6 %). Most common extra-hematologic toxicities included grade 2–3 sensory neuropathy (80 %) and fatigue (64 %). DVT was less than 10 % and no treatment-related mortalities were observed. To date, no study has confirmed the superiority of VRD over RD.
A phase 2 study has recently combined VRD with cyclophosphamide (VCRD) in previously untreated MM [40]. Responses were higher with VCRD, with at least PR rate of 96 %, and CR rate of 40 %. Longer follow-up is needed to assess a PFS and OS advantage. A phase 2 study by Kumar and colleagues found that, although the four-drug combination was associated with higher rate of VGPR (58 % vs. 51 %) and CR (25 % vs. 24 %), VRD showed to have a better toxicity profile, with a lower rate of discontinuation and treatment-related deaths. Thus VRD may be preferred in the clinical practice. However, further evaluation in a phase 3 study is necessary [41].
Recently, the addition of pegylated liposomal doxorubicin to the 3-drug regimen VRD (VRDD), was explored in a phase 1/2 trial, in which both patients eligible and not eligible for ASCT were enrolled. Patients received 4 to 8 cycles of VRDD and were allowed to undergo ASCT if they had achieved at least a PR after 4 cycles. After the first 4 cycles, the rates of CR/ near CR and ≥ VGPR were 29 and 57 %, respectively. Forty-nine patients underwent ASCT and their response was analysed three months after the transplant: the rates of CR/ near CR and ≥VGPR were 61 and 85 %, respectively. Since the achievement of a deep response (≥VGPR) with induction treatment before ASCT is associated with longer PFS and OS, the 4-drug combination VRDD may be suitable for younger patients willing to proceed to ASCT, although its role must be confirmed in a larger, phase 3 clinical trial [42].
Bortezomib-Containing Regimens
The association of bortezomib and dexamethasone (VD) showed to be an effective and safe frontline approach both in patients eligible and ineligible for ASCT [43]. In two trials [44, 45], VD combination was given as induction before ASCT. At least VGPR rate increased from 30 % before transplantation to 55–60 % after ASCT.
Chemotherapy with VAD has long been a standard induction for transplant eligible patients. A phase 3 study compared VAD regimen with VD as induction therapy before ASCT [46]. VD showed to be superior to VAD in terms of response (≥VGPR 38 % vs 15 % after induction and 68 % vs 47 % after double ASCT) and of median PFS (36 months vs 30 months), although this benefit was not statistically significant.
Cytotoxic drugs like doxorubicin and cyclophosphamide were added to VD as part of 3-drug regimens before ASCT. The randomized phase 3 HOVON-65/ GMMG-HD4 trial compared the association of bortezomib, doxorubicin and dexamethasone (PAD) with the standard induction regimen VAD; the rate of nCR/CR was significantly higher with the bortezomib schedule (34 % versus 49 %, respectively (P < 0.001)); median PFS and OS were 28 and 55 months for the VAD arm and 35 and 61 months for the PAD arm [47]. In two phase 2 studies, the addition of cyclophosphamide to bortezomib-dexamethasone (VCD or CyBorD) led to a rate of ≥VGPR that varies between 37 and 61 % [40, 48].
Of note, bortezomib-containing regimens seem to overcome the poor prognosis associated with t(4;14), del 17, and other cytogenetic abnormalities [49–52]. A phase 3 trial compared TD vs TD plus bortezomib (VTD) as induction treatment before double ASCT followed by consolidation/maintenance therapy [49]. After induction, the CR rate was 19 and 5 % in the VTD and TD arm respectively (P < 0.0001), they increased to 42 and 30 % respectively after the second ASCT (P = 0.0004). VTD confirmed to be superior to TD also after consolidation therapy, with 49 % CR rate after VTD consolidation vs 34 % after TD consolidation (P = 0.0012). Three-year PFS was longer with VTD (68 % vs 56 % P = 0.005) but the 3-year OS was similar between the two treatment groups (86 % vs 84 % respectively). PFS by subgroup analysis showed an advantage with VTD in high-risk patients, such as patients with chromosome abnormalities (del 13, t(4;14) with or without del 17) advanced ISS and older age. Most common toxicities included skin rash, peripheral neuropathy, infection and DVT, although none of them had an incidence higher than 10 %. The positive results achieved in this study provided the basis for the European Medication Agency to approve VTD combination as induction regimen in transplant-eligible patients.
According to risk-stratification provided by the Mayo Clinic group [53], VRD is suggested for high-risk patients, whereas VCD can be used for intermediate-risk patients before ASCT. Whether a more aggressive strategy should be adopted mainly depends on the importance of achieving a high CR rate and of prolonging OS. In low-risk patients, the achievement of CR seems not to be related to longer OS. In these patients, a lenalidomide-containing regimen such as Rd is suggested before ASCT, subsequently lenalidomide maintenance may be administered as well.
Elderly Patients
Patients older than 65 years, or subject with significant comorbidities, are usually considered ineligible for standard melphalan 200 mg/m2 followed by ASCT. For these patients gentler approaches, and, if necessary, age-adjusted dose modifications are required (Table 12.2).
Table 12.2
Recommended dose reductions based on patient age
Drug | Age 65–75 years | Age ≥75 years |
|---|---|---|
Dexamethasone | 40 mg weekly | 20 mg weekly |
Melphalan | 0.25 mg/kg days 1–4 every 6 weeks | 0.18 mg/kg days 1–4 every 6 weeks or 0.13 mg/kg days 1–4 every 4 weeks |
Thalidomide | 100 or 200 mg/day continuously | 50 or 100 mg/day continuously |
Lenalidomidea | 15–25 mg/day days 1–21 every 4 weeks | 10–25 mg/day days 1–21 every 4 weeks |
Bortezomib | 1.3 mg/m2 days 1, 4, 8, 11 every 3 weeks or days 1, 8, 15, 22 every 5 weeks | 1.0–1.3 mg/m2 days 1, 8, 15, 22 every 5 weeks |
Thalidomide-Based Regimens
The combination melphalan and prednisone (MP) has been considered, until recently, the standard of care in patients not eligible for transplantation.
The two-drug combination TD has been assessed in elderly patients. Although TD was more effective than standard MP, TD was also associated with a higher incidence of adverse events, treatment discontinuations, and non-disease-related mortality, mainly due to infections [54].
Six randomized studies have validated the role of thalidomide plus MP (MPT) in this setting [55–60]. The two French studies found both a PFS and an OS advantage with MPT compared to MP [55, 56] while the PFS benefit detected in the Italian study did not translate into a survival advantage [60]. The Dutch/Belgian study found differences in PFS and OS between MPT and MP [59]. No significant PFS and OS differences were reported between MPT and MP in the Nordic study, although MPT patients had improved response during the first year of treatment [58]. Grade 3–4 neutropenia (16–48 %) was the most common toxicity with MPT, and was mainly due to melphalan. Peripheral neuropathy (6–23 %) and venous thromboembolism (3–12 %) were quite frequent and they were associated with thalidomide administration. Importantly, anti-thrombotic prophylaxis is recommended when administering thalidomide [61].
Recently, an efficacy meta-analysis pooling together data from 1,685 patients enrolled in the six MPT was performed, and found that median survival was 32.7 months with MP vs 39.3 months with MPT [62]. Early deaths were more frequent with MPT during the first year of treatment, and the advantage of MPT over MP was evident with longer follow-up: the 2-year PFS was 42.5 % with MPT and 28.4 % with MP. Responses were higher in MPT patients, with at least VGPR rate of 25 % for MPT and 9 % for MP. Although this meta-analysis confirmed that MPT improved PFS and OS in previously untreated MM patients, no particular advantage was seen in patients with poor performance status or renal impairment. However, considering the positive results, MPT is today considered one of the new standards of care for elderly patients with MM.
The role of thalidomide has been also assessed in combination with cyclophosphamide and dexamethasone (CTD) [63]. Responses were improved with CTD, with an overall response rate of 32.6 % with MP vs 63.8 % with CTD (P < 0.001). The median PFS was similar between the two arms (12.4 months with MP vs 13 months with CTD) and so was also the median OS (30.6 months with MP vs 33.2 months with CTD). Of note, CTD showed to be particularly beneficial in subjects with a good cytogenetic profile by FISH. Constipation (41 %), infection (32 %), sensory neuropathy (24 %), and DVT (16 %) were common with CTD. Although thromboprophylaxis was not initially planned in this trial, it was subsequently used for patients receiving thalidomide. This dramatically decreased the rate of thromboembolic events. CTD showed to be a possible approach for selected elderly newly diagnosed MM patients, and is most beneficial in standard-risk patients by FISH analysis.
Lenalidomide-Based Regimens
The RD vs Rd trial previously mentioned included not only young patients but also elderly patients [38]. RD was associated with a higher rate of side effects than Rd, such as DVT or pulmonary embolism (26 % vs 12 %), and infections (16 % vs 9 %), particularly in patients ≥65 years. Considering the better safety profile, Rd is now to be preferred, especially in the elderly setting. Nevertheless, the use of high-dose dexamethasone is still beneficial for patients with renal failure, hypercalcemia, pain and spinal cord compression, at diagnosis.
Recently, a phase 3 trial compared the combination melphalan-prednisone-lenaldiomide followed by lenalidomide maintenance (MPR-R), with MPR and MP [64]. MPR-R prolonged the median PFS in comparison to MPR and MP (31 vs 14 vs 13 months; P < 0.001). However, in patients older than 75 years of age, MPR induction did not improve PFS as compared with MP. This may be due to the higher rate of adverse events with MPR and the need for more frequent dose modifications in older patients than in younger patients. The 3-year OS was similar between the three treatment arms (70 % vs 62 % vs 66 %). Grade 4 neutropenia was reported in 35 % of MPR-R patients and 32 % of MPR patients. Despite the recent concerns about lenalidomide-related occurrence of second primary malignancies (SPMs: the 3-year rate was 7 % with both MPR-R and MPR, and 3 % with MP), the benefits associated with MPR-R outweigh the increased risk of SPMs. Similarly to thalidomide, antithrombotic prophylaxis is recommended when patients receive a lenalidomide-containing regimen [61].
Lenalidomide combined with another alkylant agent, cyclophosphamide, plus dexamethasone (CRD) showed to be effective in a phase 2 study including both young and elderly patients [65]. Of note, CRD led to at least VGPR rate of 30 %. The most common hematologic adverse event was neutropenia, and it was manageable with cyclophosphamide dose reductions. Fatigue was the more frequent non-hematologic adverse event. No thromboprophylaxis was planned in the protocol, but it was recommended for high-risk patients, and DVT occurred in 15 % of the patients enrolled. The 2-year PFS rate was 57 % and the 2-year OS rate was 87 %.
A three-arm randomized phase 3 trial is currently ongoing to compare Rd vs MPR vs CPR in elderly MM patients [66]. The major aim is to evaluate the impact of adding an alkylator to Rd, and which one between melphalan and cyclophosphamide is the is the best. Results are still preliminary, but to date no particular advantage in PFS and OS of the triplets over the doublet has been noted.
Bortezomib-Based Regimens
The combination VD showed to be a valid option for both young and elderly patients [43]. A phase 2 trial reported at least PR rate of 90 %, with at least VGPR rate of 42, and 19 % patients in CR/ near CR after VD. In patients who did not receive ASCT, median PFS was 21 months and the median OS was not reached. Adverse events were quite limited, with few cases of grade 3–4 neutropenia and peripheral neuropathy, and no DVT, although no thromboprophylaxis was given.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree