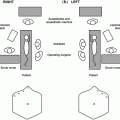
Fig. 30.1
MEN syndromes and their associated tumours. Multiple endocrine neoplasia type 1 (MEN1) is characterised by the combined occurrence of anterior pituitary, pancreatic islet and parathyroid tumours; MEN type 2A (MEN2) by parathyroid, medullary thyroid and adrenal medullary tumours; MEN type 2B (MEN3) by medullary thyroid, adrenal medulla and neuroma tumours; and MEN type 4 (MEN4) by anterior pituitary, gastroenteric neuroendocrine and parathyroid tumours
Multiple Endocrine Neoplasia Type 1 (MEN1)
Clinical Features
Patients with MEN1 develop parathyroid (95%), pancreatic islet (80%) and pituitary (30%) tumours (Fig. 30.1) [7]. A number of other syndromic tumours occur and include cutaneous angiofibromas (88%) and collagenomas (72%), adrenal cortical tumours (35%), multiple lipomas (33%), foregut carcinoids (15%), meningiomas (<10%) and facial ependymomas (<5%) [13, 14]. A progression of tumour development from hyperplasia to adenoma to carcinomas occurs in MEN1 patients [1]. The prevalence of MEN1 in the UK was estimated to be ~10 per 100,000 of the population, with an equal sex distribution [15]. Amongst the pancreatic NETs, over half are gastrinomas, a third are insulinomas, and <5% are glucagonomas, vasoactive intestinal peptide (VIP)-omas or pancreatic polypeptide (PP)-omas; and amongst the pituitary NETs, two thirds are prolactinomas, a quarter are somatotrophinomas, ~5% are adrenocorticotrophinomas, and ~5% are non-functioning adenomas [15]. MEN1 has a high degree of penetrance, such that >95% of patients develop clinical symptoms by the fifth decade [15], and children as young as 5 years old have been found to have MEN1-associated tumours [16, 17]. However, most MEN1-associated tumours develop after 10 years of age [18].
Diagnosis and Treatments of MEN1-Associated Tumours
Parathyroid Tumours
Primary hyperparathyroidism is the commonest and first manifestation of MEN1 and occurs from ~20 years of age in MEN1 patients [7]. Children with primary hyperparathyroidism may often have MEN1 [19]. Patients have hypercalcaemia, hypophosphataemia and a raised or inappropriately normal parathyroid hormone (PTH) level, with normal or increased 24 h urinary calcium excretion [1]. This is often asymptomatic and discovered on biochemical screening, but patients may also present with nephrolithiasis, peptic ulceration, polyuria, polydipsia, constipation and fatigue [7]. Surgery is the treatment of choice for primary hyperparathyroidism. Subtotal (leaving half of the smallest parathyroid gland) or total parathyroidectomy with transcervical near-total thymectomy is the recommended treatment [3, 20]. Parathyroid autotransplantation may be considered, but parathyroidectomy for hyperparathyroidism in MEN1 is associated with a high failure rate, including an image-guided approach in children with MEN1 [21], with recurrent hypercalcaemia in ~50% within a decade of surgery [22]. Randomised controlled trials in MEN1 patients are lacking and guidelines are mainly based on expert opinion [3].
Pancreatic Islet Neuroendocrine Tumours (Nets)
Anterior Pituitary NETs
Anterior pituitary tumours occur in 30–50% of MEN1 patients, with a higher incidence in female patients [23]. The clinical manifestations are related to hypersecretion of anterior pituitary hormones, or from compression of adjacent structures by an enlarging tumour, such as bitemporal hemianopia from compression of the optic chiasm, or hypopituitarism from compression of adjacent normal pituitary. Prolactinomas are the commonest pituitary tumour (~60% of pituitary NETs in MEN1 patients) and the excess prolactin secretion from lactotrophs results in secondary amenorrhea, infertility and galactorrhoea in female patients, and impotence in male patients [15]. Somatotrophinomas (~25%) secrete growth hormone from somatotrophs resulting in acromegaly. Least common amongst pituitary NETs in MEN1 patients are non-functioning adenomas (~5%) and adrenocorticotrophinomas (~5%), which secrete adrenocorticotrophic hormone (ACTH) and cause Cushing’s disease [15]. Diagnosis relies on biochemical testing for prolactin and insulin-like growth factor-1 with magnetic resonance imaging (MRI) of the pituitary fossa. Treatment of pituitary NETs is often with dopamine agonists such as bromocriptine or cabergoline for prolactinomas, somatostatin analogues for somatotrophinomas [24] or transphenoidal hypophysectomy for enlarging tumours causing mass effects [25]. Radiotherapy is available for inoperable or recurrent tumours.
Adrenal Cortical Tumours
Cortical adrenal tumours occur in 10–30% of MEN1 patients [26]. Most adrenal tumours in MEN1 patients are benign and non-functioning, and since the prevalence of adrenal tumours is ~5% in the general population, only MEN1 patients with adrenal tumours >4 cm in diameter should be assessed for adrenalectomy, as the risk of malignant transformation increases with adrenal size [14, 27]. Adrenal tumours in MEN1 may be hyperplastic, cystic, cortical adenomas, or carcinomas [28]. However, functioning adrenocortical tumours in patients with MEN1 cause hypercortisolaemia (Cushing’s syndrome) and primary hyperaldosteronism (Conn’s syndrome), which necessitate adrenalectomy [14].
Thymic, Bronchopulmonary and Gastric Nets
NETs of foregut origin occur in up to 15% of MEN1 patients and are located in the thymus, the respiratory tract and the upper gastrointestinal tract [29]. Thymic NETs are particularly aggressive in male MEN1 patients and account for significantly increased mortality [23]. Tumours may be asymptomatic and metastases may be associated with symptoms of the carcinoid syndrome (facial flushing, bronchospasm and intractable diarrhoea). Curative surgery, where possible, is the treatment of choice for thymic and bronchial carcinoid tumours. Where disease is advanced and inoperable, additional therapies include somatostatin analogues, radiotherapy and chemotherapy, but prognosis remains poor for advanced tumours [30].
Treatment of NETs in Patients with MEN1 Versus NETs in Sporadic Patients
Treatment for NETs in MEN1 patients, which include pancreatic islet NETs, anterior pituitary NETs and foregut carcinoids, is more difficult than for equivalent tumours in non-MEN1 (sporadic) patients for several reasons [31]. First, MEN1 tumours, with the exception of anterior pituitary NETs, are multiple. For example, multiple submucosal duodenal and pancreatic gastrinomas develop in MEN1 patients, thereby reducing surgical cure rates, such that at 5 years ~5% in MEN1 patients, compared to ~40% in non-MEN1 patients were disease free [32]. Second, occult metastatic disease is more prevalent in MEN1 patients with NETs than in patients with sporadic endocrine tumours. For example, metastases are present in up to 50% of patients with MEN1-associated insulinomas, whereas <10% of non-MEN1 insulinomas metastasize [33]. Third, the majority (~80%) of NETs have low proliferation rates, with a Ki-67 index <2%, and may not respond to chemotherapy or radiotherapy. Fourth, NETs in MEN1 patients are larger, more aggressive, and resistant to treatment [31]. For example, ~85% of anterior pituitary NETs in MEN1 patients, as opposed to 64% in non-MEN1 patients are macroadenomas; ~30% of anterior pituitary NETs in MEN1 patients have invaded surrounding tissue, compared to 10% in non-MEN1 patients [34]; and >45% of anterior pituitary NETs in MEN1 patients had persistent hormonal over-secretion following appropriate medical, surgical and radiotherapy treatment, compared to 10–40% in non-MEN1 patients [35]. Thus, there is a clinical need for better and alternative treatments for NETs in MEN1 patients.
Molecular Genetics of MEN1
MEN1-associated tumours have loss of heterozygosity (LOH) for the MEN1 gene located on chromosome 11q13 that encodes Menin [36, 37]. Germline mutations of the MEN1 gene coding region are present in the majority (>90%) of patients with MEN1 and most of the MEN1 mutations are inactivating, consistent with a tumour suppressor role for Menin [38]. The mutations are scattered throughout the 1830 base-pair (bp) coding region and splice sites of the MEN1 gene, and there appears to be no genotype-phenotype correlation in MEN1, with members of the same pedigree developing different MEN1-associated tumours. Menin is an ubiquitously expressed nuclear protein that has been shown to have roles in transcriptional regulation; genome stability; cell division; proliferation; cell cycle control; and apoptosis [39, 40]. More recently, the three-dimentional structure of Menin has been solved and it has been shown that Menin is a molecular adaptor coordinating the functions of multiple proteins [41]. For example, Menin has been shown to regulate the expression of cyclin-dependent kinase inhibitors (p27 and p18) via MLL-1, thereby reducing cellular proliferation [42], and to induce caspase 8 expression that promotes apoptosis [39]. Thus, loss of menin function leads to endocrine tumourigenesis in MEN1 [1].
Mouse models with loss of Men1 alleles that are representative of MEN1 in man have been developed to understand the molecular basis of tumourigenesis in MEN1 [43–45]. A conventional heterozygous knockout mouse model for MEN1 (Men1 +/− knockout mice) [43] develops parathyroid, pancreatic islet, anterior pituitary and adrenal cortical tumours, associated with an increased mortality of 30%. Accurate assessment of proliferation and apoptosis rates in these MEN1 knockout mice have been investigated to understand MEN1-associated neuroendocrine tumourigenesis [46]. Using this data, mathematical modelling of NET growth rates (proliferation minus apoptotic rates) predicted that in Men1 +/− mice, only pancreatic β-cells, pituitary lactotrophs and somatotrophs could develop into tumours within a murine lifespan. Men1 +/− tumours had low proliferation rates (<2%), second-order growth kinetics, and the higher occurrence of insulinomas, prolactinomas and somatotrophinomas in MEN1 was consistent with a mathematical model for NET proliferation [46]. Translational studies of gene replacement therapy in Men1 +/− mice generated menin expression in anterior pituitary tumours, and significantly reduced tumour cellullar proliferation. It demonstrated proof of concept for MEN1 gene replacement therapy for inhibiting NET growth in MEN1 [44]. The development of such MEN1 gene therapy is likely to be of use in MEN1 patients for the treatment of anterior pituitary, pancreatic islet and thymic NETs. Finally, evaluation of a new somatostatin analogue, pasireotide, was shown in conditional Men1 +/− mice to have anti-proliferative and pro-apoptotic effects in insulinomas [47] and in conventional Men1 +/− mice increased survival by ~20%, inhibited tumour growth, and reduced the number and volume of pancreatic islet and anterior pituitary tumours, thereby indicating its potential use to treat pancreatic and pituitary NETs [48]. Thus, new treatments may be developed for MEN1 patients following these recent advances in pre-clinical science.
Multiple Endocrine Neoplasia Type 4
Clinical Features of the MEN4 Syndrome
MEN4 was only recently described, and patients develop parathyroid (81%) and anterior pituitary tumours (42%) [8, 9, 11, 49]. Patients may also develop gastric and bronchial carcinoids or gastrinomas that cause Zollinger-Ellison syndrome (Fig. 30.1). A recent case report described a possible association with meningioma and papillary thyroid carcinoma in a patient with a rare CDKN1B gene variant [50]. MEN4 patients harbour heterozygous mutations in the CDKN1B tumour suppressor gene that encodes a cyclin-dependent kinase inhibitor, p27, which is involved in cellular proliferation. Parathyroid tumours have developed from 46 years of age, and pituitary tumours from 30 years old in MEN4 patients. Cases in children may be identified in known MEN4 families with genetic and biochemical screening and radiological follow-up.
Diagnosis and Treatments of MEN4-Associated Tumours
Parathyroid Tumours
Primary hyperparathyroidism occurred in 10 of 12 index MEN4 patients and parathyroidectomy was curative. Standard pre-operative localisation of symptomatic patients with concordant sestamibi and ultrasound studies permited minimally invasive parathyroidectomy in this situation [51]. No evidance of parathyroid malignancy has been described.
Anterior Pituitary NETs
In 5 of 12 index MEN4 cases, anterior pituitary tumours developed. Two patients had clinical features of acromegaly due to somatotrophinomas, one had a prolactinoma, one had Cushing’s disease due to a corticotrophinoma, and one patient had a non-functioning tumour. Transsphenoidal surgery is the treatment of choice for pituitary adenomas, but is not always curative. Prolactinomas can be successfully treated with dopamine agonists, and radiotherapy may be used after surgical treatment in recurrent disease. In one MEN4 patient with a somatotrophinoma, there was local invasion, cellular atypia and a high mitotic index, indicating an aggressive tumour. However, until more cases are described, expert multidisciplinary team management is recommended.
Molecular Genetics of MEN4
MEN4 patients have inactivating mutations in the CDKN1B gene located on chromosome 12p13 and its 2 exons encodes a 196 amino acid cyclin-dependent kinase inhibitor referred to as p27 [8]. So far, 12 CDKN1B mutations have been described that are located throughout the gene and its promotor region. Furthermore, somatic mutations of CDKN1B have been reported in sporadic parathyroid adenomas [52]. P27 is an ubiquitously expressed nuclear protein of the cyclin-dependent kinase inhibitor family. It regulates cell cycle progression from G1 to S phase by inactivating cyclin A/E cyclin-dependent kinase (CDK) 2 complexes, so that cell division is blocked. Phosphorylation of p27 enables its export from the nucleus for cytoplasmic ubiquitination and degradation by the proteosome. Inactivating mutations of CDKN1B in MEN4: reduce the cellular expression of p27; alter its intracellular location; or disrupt its ability to interact with partners such as CDK2. Syndromic tumours have reduction or loss of p27 expression, indicating that loss of the tumour suppressor function of p27 causes tumourigenesis in MEN4 patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree