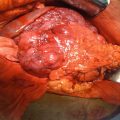
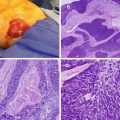
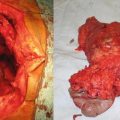
Fig. 25.1
Patients who inherited the menin mutation have an inactivated allele and a functioning allele (heterozygous). Because the functioning allele is a functioning tumor suppressor gene, these patients do not have cancer, although they are at an increased risk of developing it. When the second functioning allele is inactivated by epigenetic silencing, errors in chromosomal segregation, deletions, etc. (loss of heterozygosity), this predisposes the patient to developing tumors (Courtesy of Quyen D. Chu, MD, MBA, FACS)
More than 1,300 different germline mutations have been identified thus far and most of these mutations are either frameshift or nonsense mutations. Germline mutations in the menin gene are found in over 75 % of patients with familial forms of MEN 1. About 30 % of sporadic type MEN 1 patients demonstrate the mutation, suggesting that other proteins may play a role in the development of the syndrome.
Loss of function of the menin gene has also been found in other sporadic endocrine tumors such as parathyroid adenomas, bronchial carcinoids, and gastrinomas; this observation further supports the importance of menin in normal endocrine organ tumor surveillance.
Clinical Presentation of MEN 1
The most common initial presenting symptom is often related to the sequelae of primary hyperparathyroidism. Because symptoms of primary hyperparathyroidism (PHPT) in patients with MEN 1 syndrome are similar to patients with sporadic PHPT, the diagnosis of MEN 1 is often overlooked and delayed for several years. Such patients may present with mild hypercalcemia, renal stones, peptic ulcer disease, and depression (Table 25.1). Nearly all patients with MEN 1 will eventually develop PHPT; there is an 80–100 % penetrance by the age of 50 [1].
Table 25.1
Conditions and symptoms seen in patients with hyperparathyroidism
Nephrolithiasis | Depression |
Osteopenia | Fatigue |
Pancreatitis | Nausea and vomiting |
Gout | Dyspepsia |
Peptic ulcer disease | Polyuria |
Weight loss | Muscle aches |
Lethargy | Pruritus |
In contrast to sporadic PHPT, which tends to manifest as single gland adenoma in upward of 85 % of cases, the PHPT seen in MEN 1 patients is typically multigland hyperplasia. The average age of onset in MEN 1 is in the third decade compared to the sixth decade for sporadic lesions [2]. Despite the prevalence of hyperparathyroidism in MEN 1 patients, parathyroid carcinoma remains rare.
The second most common manifestation of MEN 1 is the pancreatic neuroendocrine tumor (PNET) origin. These tumors can develop in up to 60 % of patients over the age of 40 [3, 4]. The morbidity associated with these tumors depends on the tumor type. Gastrinomas account for over 50 % of PNET lesions in MEN 1. Associated with persistent gastric acid hypersecretion and a predisposition to refractory ulcer disease, these are usually small and multicentric at the time of diagnosis and are found almost exclusively in the duodenum. Nearly half of gastrinomas are malignant at the time of diagnosis and often have lymph node or hepatic metastases.
Insulinomas are the second most common tumor of PNET origin (10–20 %) with a morbidity that is related to profound hypoglycemia. Besides gastrinomas and insulinomas, nonfunctioning neuroendocrine tumors have been recently reported to occur in up to 55 % of patients when screened with endoscopic ultrasound. Although they are referred to as nonfunctioning, these neuroendocrine tumors can secrete low levels of polyamines without having any associated symptoms. Glucagonomas, vasoactive intestinal peptide tumors (VIPomas), and somatostatinomas have all been reported, though in much less frequency. In glucagonomas, necrolytic migratory erythema (NME) is the pathognomonic dermatologic lesion [5].
Treatment of PNET tumors generally involves medical control of symptoms, while operative intervention is somewhat controversial, except for insulinomas. The prognosis of malignant PNETs tends to be more favorable in MEN 1 patients than that seen in sporadic cases; for MEN 1 patients, the mean survival is approximately 15 years after diagnosis compared to 5 years for patients with sporadic tumors. However, these results may be due to biases related to earlier diagnosis.
Pituitary tumors are seen in up to 50 % of patients diagnosed with MEN 1 [6]. The vast majority are microadenomas, which are defined as being smaller than 10 mm. The mean age at diagnosis is late 30s to early 40s. Prolactinoma is the most common functional tumor followed by growth hormone secreters and nonfunctional tumors, although somatotropinomas and corticotropinomas have also been reported. Pituitary tumors associated with MEN 1 tend to progress to macroadenomas and become more resistant to medical therapy over time.
Diagnosis and Screening
Genetic testing for the MEN1 gene mutation and further workup should be initiated in those patients that demonstrate any two of the three common endocrine tumors (hyperparathyroidism, pituitary tumor, PNET tumors). Patients with a family history of MEN 1 who have an associated endocrine tumor should also undergo genetic testing. Genetic testing of the offspring of MEN patient should begin at an early age. Some investigators recommend screening of individuals who have early hyperparathyroidism (under age 30), gastrinoma at any age, or multifocal pancreatic neuroendocrine tumors (PNET).
In those who harbor the MEN 1 mutation, it is recommended that they should undergo yearly screening for all of the common MEN 1-related tumors starting at the age of 5 years (Table 25.2). Any biochemical abnormalities should prompt further radiologic investigation such as a magnetic resonance imaging (MRI) of the brain for suspected pituitary tumors or computed tomography (CT)/ultrasound for PNET-related tumors. Unfortunately, genetic mutations are detected in only 75 % of patients with MEN I and early detection has yet to demonstrate an improvement in morbidity or mortality in MEN 1 patients.
Table 25.2
Screening recommendations for MEN 1
Hyperparathyroidism | Intact PTH and serum calcium—yearly starting at age 8 |
|---|---|
Pituitary tumor | Serum prolactin and insulin-like growth factor—yearly starting at age 5 |
Brain MRI based on abnormal result | |
Gastrinoma | Fasting serum gastrin yearly starting at age 20 |
Abdominal CT based on result | |
Insulinoma | Fasting insulin and glucose starting at age 5 |
Abdominal CT based on result |
Treatment
As the involved endocrine organs vary greatly from patient to patient, so does the therapy for MEN 1-affected individuals. True surgical cures are very difficult to achieve, and any intervention is typically guided toward alleviation of medically resistant symptoms and prevention of progression toward malignancy and metastatic disease. The following are recommendations for treating the most common MEN 1-related disorders.
Management of Hyperparathyroidism in MEN 1
As mentioned, nearly all patients with MEN 1 will develop hyperparathyroidism, often as the primary presenting symptom of the disorder. Confirmatory diagnosis is typically laboratory based with elevated levels of parathyroid hormone in the presence of hypercalcemia.
Much controversy surrounds the timing of intervention as well as the type of resection. Earlier intervention at the time of diagnosis limits the deleterious effects of long-standing hypercalcemia such as osteopenia, nephrolithiasis, ulcer disease, etc. but at the cost of a higher likelihood of recurrence and need for reintervention. Most patients will recur over time, often requiring further resection of any remaining parathyroid tissue. The risk of recurrence increases over time with recurrence rates being well over 50 % at 10 years in patients who had undergone subtotal or total parathyroidectomy with reimplantation surgery [9]. Given the high rate of recurrence over time, the goal of parathyroidectomy should be to achieve the longest durable remission time from hyperparathyroidism in the safest fashion for the patient.
No consensus exists with regard to whether a subtotal 3½ gland excision should be performed or a total parathyroidectomy with reimplantation. The general benefit of a subtotal resection is the low risk of developing profound hypocalcemia. However, the disadvantage with this approach is the greater risk of developing earlier recurrence and the need for reoperation in the neck. Total parathyroidectomy and reimplantation in the forearm carries a higher incidence of recalcitrant hypocalcemia but with the benefit of a less taxing operation at the forearm to remove implanted parathyroid tissue for recurrent disease.
Prior to planning any operative resection of the parathyroids, a thorough examination and evaluation of the thyroid gland should be undertaken because of the high rate of having synchronous thyroid tumors. This includes obtaining biochemical and ultrasound analysis. There is evidence that performing a cervical thymectomy at the initial resection decreases recurrence rates as well as reduces the risk of developing thymic carcinoids, a situation that can be seen in up to 10 % of MEN 1 patients [1].
Management of Pancreatic Neuroendocrine Tumors (PNET) in MEN 1
Over 60 % of patients with MEN 1 will have a PNET over their lifetime. The treatment of these tumors is predicated on whether the lesion is a functional or nonfunctional tumor, the most common being gastrinoma followed by insulinoma as previously discussed.
As gastrinoma accounts for the majority of these lesions, therapy traditionally has been medical symptom control with the use of H2 blockers and proton pump inhibitors. It is important to remember that surgical intervention should be delayed until after hypercalcemia has been addressed as calcium plays a role in acid hypersecretion. Correction of the hypercalcemic state often leads to a reduction of serum gastrin levels. As gastrinomas are often multifocal, surgical cures are rare and often require a subtotal pancreatectomy, duodenal exploration, as well as regional lymph node dissection [10]. Surgical interventions should be reserved for malignant gastrinomas or those with disease refractory to medical management.
In contrast to gastrinoma, insulinoma often requires surgical resection and is rarely malignant though symptomatic control is often more difficult. These lesions can typically be treated effectively with surgical enucleation (either with open or laparoscopic technique) and are found almost exclusively within the pancreatic parenchyma.
As with sporadic PNETs, there is a role for surgical debulking of tumor mass in all types of PNETs, and the goal is to achieve symptom control rather than surgical cure. In those patients in whom surgery is not possible, medical therapy including diazoxide, sunitinib, somatostatin, or other therapies can be employed for palliation.
Management of Pituitary Neoplasms in MEN 1
Therapy of associated pituitary neoplasm is typically directed toward controlling symptoms such as headaches and vision changes due to the mass effect of the tumor. Pituitary tumors associated with MEN 1 can also exert hormonal effects, the most common tumor being prolactinoma, followed by growth-hormone- secreting tumors and adrenocorticotropic-hormone (ACTH)-secreting tumors. The first-line therapy of prolactinoma is medical management, employing dopamine agonists such as bromocriptine or cabergoline. For growth-hormone-secreting tumors and ACTH-secreting tumors, as well as prolactinomas that are refractory to medical therapy, a transsphenoidal hypophysectomy offers a safe and effective alternative to medical management. Somatostatin analogs and/or radiation are sometimes employed with some success as primary medical therapy or for recurrences of these tumors.
Multiple Endocrine Neoplasia Type 2
Multiple endocrine neoplasia type 2 (MEN 2) includes two subtypes—A and B—as well as a third entity, familial medullary thyroid carcinoma (FMTC), all linked by mutations of the RET proto-oncogene and a tendency for the development of medullary thyroid cancers (MTC) (Table 25.3). MTC was first described in 1959 by Hazard et al. [11]. Two years later, Sipple described a patient in which MTC and pheochromocytoma were found implying a common linkage [12]. It was not until 1968 that a report of a family with MTC, pheochromocytoma, and hyperparathyroidism was reported [13]. This syndrome was later termed “Sipple syndrome” now known as MEN 2A.
Table 25.3
Manifestation of clinical features by MEN 2 subtypes
Subtype | Hyperparathyroidism | Pheochromocytoma | MTC |
|---|---|---|---|
MEN 2A | 20–30 % | 50 % | 95 % |
MEN 2B | Rare | 50 % | 100 % |
FMTC | 0 % | 0 % | 100 % |
MTC is a rare form of sporadic thyroid cancer arising from the parafollicular C cells and accounts for approximately 5 % of all thyroid malignancies. MTC tends to be more aggressive than the more common thyroid malignancies—papillary, follicular—with a predilection for lymphatic spread. Calcitonin is the product of the parafollicular C cells, and elevated serum levels can be seen in advanced disease. It can also be used as a surveillance modality after a thyroidectomy. A more thorough discussion of MTC can be found in Chap. 23, Thyroid Cancer.
Unlike MEN 1 which can have high variability in the timing as well as the types of tumors seen in the disorder, MEN 2 tends to be more predictable and early diagnosis by genetic testing has resulted in a significant reduction in the morbidity and mortality associated with the disease.
Genetics
Mutations of the RET proto-oncogene on chromosome 10 leading to MEN 2 were first discovered in 1993 [14]. The mutations were found to encode for a transmembrane tyrosine kinase receptor involved in the processes of cell migration, division, and proliferation. RET is normally expressed in neural crest-derived tissues and is key to the normal embryological development of these derived organs. Under-expression of RET has been associated with neonatal Hirschsprung’s disease with aganglionogenesis leading to colonic dysfunction. Upregulation and activation leads to MEN 2 and FMTC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree