Treatment arms
Primary end point
Secondary end points
MAGIC [3]
Surgery alone
Epirubicin 50 mg/m2 day 1
5-year OS
5-year PFS
Cisplatin 60 mg/m2 day 1
HR 0.75
HR 0.66
5-FU 200 mg/m2 daily
95 % CI 0.6–0.93
95 % CI 0.53–0.81
Every 21 days ×3 cycles
P < 0.001
Surgery
Rate of resection
Epirubicin 50 mg/m2 day 1
79.3 % vs. 70.3 % (p = 0.03)
Cisplatin 60 mg/m2 day 1
5-FU 200 mg/m2 daily
Every 21 days ×3 cycles
French FNCLCC/FFCD [5]
Surgery alone
5-FU 800 mg/m2 days 1–5
5-year OS
Disease-free survival
Cisplatin 100 mg/m2 day 1
38 % vs. 24 % (HR 0.69; 95 % CI 0.5–0.95; p = 0.02)
34 % vs. 19 % (5-year rate: 34 % v 19 %; HR, 0.65; 95 % CI, 0.48–0.89; P = 0.003)
Every 28 days ×2–3 cycles
R0 resection
84 % vs. 73 %
Surgery
5-FU 800 mg/m2 days 1–5
Cisplatin 100 mg/m2 day 1 or 2
Every 28 days ×3–4 cycles
EORTC 40954 [7]
Surgery alone
Cisplatin 50 mg/m2 on days 1, 15, 29
OS
Rate of resection
5-FU 2,000 mg/m2 continuous intravenous infusion over 24 h on days 1, 8, 15, 22, 29, 36
Trial terminated prematurely
81.9 % vs. 66.7 % (p = 0.036)
Leucovorin 500 mg/m2 over 2 h every 48 days ×2 cycles
PFS
Trial terminated prematurely
Surgery
A number of studies have evaluated the benefit of chemotherapy both in the neoadjuvant/perioperative as well as postoperative settings. The rationale behind adding chemotherapy in the neoadjuvant setting is potential downstaging of cancer by early exposure to chemotherapy in chemosensitive cases and improving patient selection by sparing surgery in patients that are at high risk of metastasis as micrometastatic disease may become evident after chemotherapy but prior to surgery. A possible disadvantage is delaying surgery and hence resectable disease may become unresectable in the interim.
A pivotal trial, MAGIC, conducted by Medical Research Council (MRC) in the United Kingdom, evaluated the role of perioperative chemotherapy in combination with surgery. A total of 503 patients were randomly assigned to either surgery alone or surgery with three preoperative and three postoperative 21-day cycles of chemotherapy consisting of epirubicin (50 mg/m2 day 1), cisplatin (60 mg/m2 day 1), and 5-fluorouracil, 5-FU (200 mg/m2 daily) also known as ECF [3]. Eligibility criteria required the presence of T2 or more advanced biopsy-proven adenocarcinoma with good performance status. Seventy-four percent had gastric, 11 % distal esophageal, and 15 % had GEJ cancer. The trial demonstrated benefit of adding chemotherapy with significant improvement in 5-year survival (36 % vs. 23 % hazard ratio [4], 0.75; 95 % confidence interval (CI), 0.6–0.93 in favor of combined modality arm) as well as progression-free survival (HR 0.66, 95 % CI 0.53–0.81, p < 0.001 in favor of combined modality arm). Additionally, increased rate of curative resection was seen in the combined modality arm (79.3 % in combined modality arm and 70.3 % in surgery-only arm, p = 0.03). Hematologic toxicity with grade 3 and 4 neutropenia was reported in 23 %, and the incidence of non-hematologic grade 3 and 4 toxicities was not very high (12 %) demonstrating an acceptable toxicity profile, but only 42 % of the patients assigned to the combined modality arm were able to complete all therapy. This highlights the decreased tolerance to chemotherapy in the postoperative setting. This trial established perioperative chemotherapy as standard of care for operable gastric cancer in Europe.
Another trial demonstrating the benefit of perioperative chemotherapy is the French FNLCC/FFCD multicenter trial [5]. A total of 224 patients were randomized to surgery alone or surgery with perioperative chemotherapy consisting of infusional 5-FU (800 mg/m daily for 5 days) and cisplatin (100 mg/m2 on day 1 or 2) every 28 days with two or three cycles delivered preoperatively and three or four cycles given postoperatively for a total of six cycles. Of the 224 patients with stage II or higher resectable disease enrolled in this trial, 55 had gastric 144 GEJ and 25 had distal esophageal cancer. This trial also demonstrated an improvement in 5-year survival (38 % vs. 24 %, Fig. 9.1) and disease-free survival (34 % vs. 19 %) with the addition of chemotherapy. Additionally, the rate of R0 resection also improved with addition of perioperative chemotherapy (84 % vs. 73 %). Like the prior study, only 50 % of patients were able to receive therapy postoperatively.
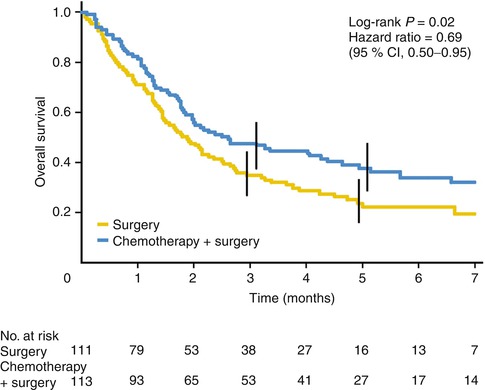

Fig. 9.1
Kaplan-Meier curve showing overall survival from date of random assignment (From Ychou et al. [5] with permission)
In contrast, an EORTC randomized trial failed to show any benefit of adding preoperative chemotherapy to surgery. In the EORTC 40954 trial, a total of 144 of the planned 360 patients with stage III and IV gastric and GEJ adenocarcinoma were randomized to surgery or preoperative chemotherapy consisting of two 48-day cycles of cisplatin (50 mg/m2 on days 1, 15, and 29) and leucovorin with FU (leucovorin 500 mg/m2 over 2 h followed by FU 2,000 mg/m2 continuous infusion over 24 h on days 1, 8, 15, 22, 29, and 36) [6, 7]. The trial was stopped early due to poor accrual and failed to show a survival benefit with the addition of chemotherapy. Analysis of the accrued patients demonstrated an improvement in the R0 resection rate (81.9 % vs. 66.7 %, p = .036) and a higher incidence of postoperative complications in the chemotherapy arm (27 % vs. 16 %).
Adjuvant Chemotherapy (Table 9.2)
Table 9.2
Adjuvant chemotherapy
Treatment arms | Primary end point | Secondary end points | |
|---|---|---|---|
ACTS-GS [16] | |||
Surgery alone | Surgery | Overall survival at 5 years: 72 % vs. 61 % (HR 0.68; 95 % CI 0.52–0.87; p = 0.003) | Relapse-free survival at 5 years 65.4 % vs. 53.1 % (HR 0.653; 95 % CI 0.537–0.793) |
S1 80–120 mg daily for 4 weeks every 6 weeks for 1 year | |||
CLASSIC [13] | |||
Surgery alone | Surgery | Disease-free survival, 3 years: 74 % vs. 59 % (HR 0.56, 95 % CI 0.44–0.72, p < 0.0001) | Overall survival, 3 years: 78 % vs. 69 %, HR 0.66, 95 % CI 0.51–0.85 |
Capecitabine 1,000 mg/m2 twice daily in days 1–14 | |||
Oxaliplatin 130 mg/m2 on day 1 every 21 days for 8 cycles | |||
A number of trials have been conducted to assess role of chemotherapy in the adjuvant setting for gastric cancer and have failed to show any survival benefit [6, 8–12]. Two large phase III studies, ACTS-GS and CLASSIC conducted in Japan and East Asia (South Korea, Taiwan and China), respectively, demonstrated a significant survival benefit with adjuvant chemotherapy establishing adjuvant chemotherapy after adequate surgery as standard of care in these regions. In the ACTS-GS trial, 1,059 patients with stage II and III gastric cancer were randomized to surgery (included a D2 lymphadenectomy in both arms) with and without adjuvant therapy with S1 administered at a dose of 80–120 mg daily for 4 out of 6 weeks for one year. S1 is an oral fluoropyrimidine that consists of three components: ftorafur (tegafur), gimeracil (5-chloro-2,4 dihydropyridine), and oteracil (potassium oxonate). The addition of adjuvant S1 led to an improvement in 5-year overall survival from 61 to 72 %. Relapse-free survival at 5 years was also found to be significantly better at 65.4 % in the S1 group compared to 53.1 % in the surgery-only group (HR 0.653; 95 % CI, 0.537–0.793).
In the multicenter CLASSIC trial, the adjuvant chemotherapy regimen consisted of capecitabine (1,000 mg/m2 twice daily in days 1–14) plus oxaliplatin (130 mg/m2 on day 1) given every 21 days for 8 cycles. A total of 1,035 patients with stage II, IIIA, or IIIB gastric cancer patients were randomly assigned to surgery with D2 lymphadenectomy alone vs. surgery followed by adjuvant chemotherapy. At a median follow-up of 34 months, there was a significant improvement in 3-year disease-free survival in the chemotherapy arm (74 vs. 59 % in chemotherapy vs. surgery-alone arm, HR for death 0.56, 95 % CI 0.44–0.72, p < 0.0001) as well as a marginally significant improvement in OS at the time of initial report in 2012 (83 vs. 78 %, HR 0.72, 95 % CI 0.52–1.00) with more robust improvement in OS with longer follow-up (78 vs. 69 %, HR for death 0.66 %, 95 % CI 0.51–0.85) [13, 14]. On evaluation of tolerance and toxicity, grade 3 and 4 adverse events were reported in 56 % of patients in the combined modality group and in only 6 % of patients in the surgery-only group. Only 67 % of patients were able to complete all 8 planned cycles of chemotherapy with 90 % of patients requiring dose modifications.
High survival rates even in the surgery-alone arms in both these trials have led to a debate about the pertinence of this data to western population. Epidemiological and clinical variations in gastric cancer between eastern and western populations have led to a hypothesis that there is a difference in biology of gastric cancer and hence variable response to therapies in different parts of the world.
Additionally a recent meta-analysis also supported the role of adjuvant chemotherapy for resectable gastric cancer [15]. Based on these studies, adjuvant chemotherapy only is the standard of care in East Asia.
Role of Radiation
Radiation in most cancers has been shown to have a role in improving local disease control. Based on the natural history of gastric cancer, local recurrence has been reported in a high proportion of cases, which led evaluation of radiation with or without chemotherapy in addition to surgical resection for patients with potentially curable disease.
In one of the earlier studies conducted by the British Stomach Cancer Group (Table 9.3), patients were randomly assigned to surgery alone, surgery followed by 45–50 Gy of radiation, and surgery followed by chemotherapy consisting of eight courses of 5-FU, doxorubicin, and mitomycin [9]. This trial demonstrated an improvement in the local control rate with the addition of adjuvant radiation (27 % vs. 10 % in favor of radiation), but no significant difference was observed in OS between the three arms.
In another study conducted by the European Organization for Research and Treatment of Cancer (EORTC), 115 patients underwent surgery and then were randomly assigned to four different groups in the adjuvant setting [17]. The first group received 55.5 Gy of postoperative radiation only, while the other three groups received radiation in combination with short-term 5-FU, long-term 5-FU, and both short-term and long-term 5-FU. Unadjusted analysis showed a significant difference in OS between the four groups, but when other pertinent prognostic factors were added to the model, there was no significant difference in survival.
A number of trials have evaluated the role of radiation in combination with chemotherapy in the adjuvant setting. In the Intergroup 0116 study, 556 patients with stage IB through IV gastric or gastroesophageal cancer were randomized to observation vs. adjuvant chemoradiation after surgery [18]. Chemoradiation consisted of an initial 28-day cycle of 5-FU and leucovorin given on days 1–5, followed by 5-FU based concurrent chemoradiation for 5 weeks (radiation dosage was 45 Gy at 1.8 Gy per day, given 5 days per week along with 5-FU on first 4 and last 3 days of radiation), break for 1 month, and then two additional cycles of chemotherapy. At a 4-year median follow-up, there was a significant difference in median survival (36 vs. 27 months), 3-year disease-free survival (48 % vs. 31 %), OS (50 % vs. 41 %), and local failures (29 % vs. 19 %) in favor of the tri-modality therapy arm. With a longer 10-year median follow-up, OS continued to be significantly better in the combined modality arm (43 % vs. 28 %, HR 1.32, 95 % CI 1.10–1.60, p = 0.0046) [19]. This study established the role of concurrent chemoradiation as an effective adjuvant regimen but has been a focus of considerable criticism as more than half of the patients enrolled in this study underwent inadequate D0 lymph node dissection and only 10 % underwent D2 lymph node dissection.
In a CALGB 80101 study, adjuvant combination chemotherapy with chemoradiation based on the INT 0116 regimen was compared with a more intense postoperative regimen consisting of one cycle of ECF followed by concurrent chemoradiation and 2 more cycles of dose-reduced ECF. The rationale was that more intensive systemic chemotherapy may translate to better OS. As reported in the American Society of Clinical Oncology meeting in 2011, there was no difference in survival between the two arms [20]. To evaluate an alternative chemotherapy backbone with concurrent radiation, a trial conducted at MD Anderson Cancer Center evaluated a neoadjuvant regimen consisting of induction chemotherapy for 2 cycles (5-FU 750 mg/m2/day days 1–5, cisplatin 15 mg/m2/days 1–5, and paclitaxel 200 mg/m2 day 1) followed by concurrent chemoradiation (45 Gy over 5 weeks, 5-FU 300 mg/m2/day 5 days/week, and paclitaxel 45 mg/m2 on days 1, 8, 15, 22, and 29) and then surgery. Of the 41 patients enrolled, the majority had proximal gastric cancer (83 %), 40 patients underwent surgery, and 78 % had an R0 resection. Pathological complete and partial response (defined as less than 10 % residual cancer cells) was seen in 20 and 15 % of patients, respectively. At a median follow-up of 36 months, OS was found to be significantly associated with pathological response (both complete and partial, p = 0.006) in addition to R0 resection, postsurgical nodal positivity, N stage, and T stage [21].
In the Radiation Therapy Oncology Group (RTOG) 0114 randomized phase II study, two postoperative adjuvant regimens consisting of induction chemotherapy followed by concurrent chemoradiation were evaluated. A total of 87 patients were randomly assigned to receive two cycles of chemotherapy consisting of paclitaxel/cisplatin/5-FU (PCF) followed by concurrent chemoradiation with paclitaxel and 5-FU or paclitaxel/cisplatin (PC) for two cycles followed by concurrent chemoradiation with paclitaxel and cisplatin. The PCF arm was closed early due to excessive gastrointestinal toxicity and the trial failed to achieve its primary end point of improvement in 2-year DFS and, hence, further evaluation in a phase III study was not recommended [22].
A recent phase III ARTIST trial conducted in Korea provided a direct comparison of chemotherapy and chemoradiation in the adjuvant setting after surgery with D2 lymph node dissection [23]. Four hundred and fifty-eight patients were randomly assigned postoperatively to either chemotherapy arm consisting of capecitabine and cisplatin (capecitabine 2,000 mg/m2/day 1–14 and cisplatin 60 mg/m2 on day 1, repeated every 3 weeks) for 6 cycles or the chemoradiation arm consisting of two cycles of chemotherapy with capecitabine and cisplatin as above followed by concurrent chemoradiation for 5 weeks (capecitabine 1,650 mg/m2/day with radiation, 1.8 Gy/day for 5 days/week for a total of 45 Gy) followed by two additional cycles of chemotherapy. Though DFS was not significantly prolonged with addition of radiation for the entire study group (p = 0.0862), a subgroup of patients with surgical pathological lymph node involvement experienced superior DFS in the chemoradiation arm (p = 0.0365). Based on these results a subsequent trial ARTIST II will evaluate the role of chemoradiation in node-positive disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree