Better understanding of the molecular biology of renal cell carcinoma (RCC) has led to the development of several targeted anti-cancer agents, several of which have since received approval for treatment of advanced disease. Two of these, the intravenous agent temsirolimus and the oral everolimus, exhibit antitumor effects through inhibition of the mammalian target of rapamycin (mTOR) pathway. This article reviews their mechanisms of action in the context of the current understanding of RCC pathophysiology, the clinical data leading to their approval, class-specific toxicities, potential molecular mechanisms behind treatment resistance and novel treatment approaches for RCC that incorporate mTOR blockade.
Better understanding of the genetics and molecular biology of renal cell carcinoma (RCC) has led to the development of several targeted agents, six of which have previously received US Food and Drug Administration (FDA) approval for treatment of advanced disease based on large international phase III trials.
Four of these compounds (sunitinib, sorafenib, pazopanib, and bevacizumab) inhibit tumor angiogenesis through blockade of vascular endothelial growth factor (VEGF). A second class of agents includes the intravenously administered temsirolimus and the oral compound everolimus, which both exhibit antitumor effects through inhibition of the mammalian target of rapamycin (mTOR). The mechanisms of action, clinical data leading to approval, clinical use of mTOR inhibitors, as well as current understanding of the molecular mechanisms behind resistance to this class of drugs are reviewed here.
Biology and mechanism of action
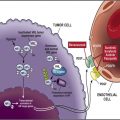
The phosphatidyl-inositol-3 kinase (PI3K)/Akt/mTOR pathway is a molecular signaling axis with key impact on various integral cellular functions, including protein synthesis, glucose metabolism, cellular migration, and cell survival. It has been implicated in promoting tumor growth. The pathway is affected by growth factors (epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1), fibroblast growth factor [FGF]), hormones (estrogen, thyroid hormones), vitamins, integrins, intracellular calcium, and the Ras-dependent mitogen-activated protein kinase (MAPK) pathway. Binding of insulin or insulin-like growth factors to their respective receptors leads to recruitment of PI3K. Once activated, PI3K converts phosphatidylinositol-4,5-phosphate (PIP2) to phosphatidylinositol-3,4,5-phosphate (PIP3), which in turn activates Akt/PKB, a serine/threonine kinase. PI3K signaling is inhibited by action of the tumor suppressor phosphatase and tensin homolog (PTEN), which negatively affects formation of PIP3, thus limiting Akt activity. Activated Akt promotes several biological processes through phosphorylation of various downstream targets and indirectly activates mTOR, a high-molecular-weight serine threonine kinase. Specifically, activated Akt phosphorylates tuberous sclerosis complex (TSC)2, leading to disassociation of the TSC1/TSC2 heterodimer complex, and inhibiting the ability of TSC2 to act as a GTPase-activating protein. This allows Rheb (Ras homologue enriched in the brain), an activator of mTOR, to remain GTP-bound and thus active. In addition to and independent of upstream signaling through PI3K, mTOR integrates other stimuli including nutrients (also through TSC1/2 and Rheb), cellular energy levels, and cellular stress. In normal cells, this helps to coordinate cell-cycle progression from G1 to S phase; in tumorigenesis, however, deregulation of components in this intricate system promotes growth and proliferation of malignant clones.
Once activated, mTOR exerts its action via protein synthesis and affects various cellular functions including cell growth, proliferation, angiogenesis, and metabolism. The latter includes effects on glucose and lipid control, which has implications for therapeutically targeting mTOR. mTOR has been found to act through two structurally and functionally distinct multiprotein signaling complexes, mTOR complex 1 (mTORC1, comprising mTOR and a scaffolding protein termed regulatory-associated protein of mTOR [raptor]) and mTOR complex 2 (mTORC2, comprising mTOR and a scaffolding protein termed rapamycin insensitive companion of mTOR [rictor]).
Activation of mTORC1 takes place indirectly through inhibition of its repressor TSC2 as outlined above. The main downstream targets of mTORC1 are the ribosomal S6 kinase (p70S6K) and the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1). Phosphorylation of 4E-BP1 through mTORC1 results in the release of 4E-BP1 from the eukaryotic translation initiation factor (eIF4E), which is then freed to interact with eIF4 G and other proteins to assemble the mammalian ribosome initiation complex eIF4F. In cancer cells, 4E-BP1 phosphorylation eventually results in the initiation of translation. The activation of mTORC1 downstream targets, through its effect on protein synthesis, modulates activity of cell-cycle–regulating proteins, hypoxia-inducible factor 1 alpha (HIF1α), FGF, VEGF, signal transducer and activator of transcription 3 (STAT3), cyclin-D, and c-Myc.
In the pathogenesis of RCC, regulation of HIF, a known oncologic driver of the disease, appears specifically relevant and possibly central to the antitumor effects of mTOR inhibitors.
The details of mTORC2 functions are less well understood. Once active, mTORC2 phosphorylates the hydrophobic motif of the AGC kinase family, including Akt (S 473 ), thereby inducing Akt activation. Other possible downstream targets with effect on cell survival, proliferation, and cytoskeleton include the Forkhead box, class O (FOXO) class of transcription factors, protein kinase C alpha (PKCα), and the serum- and glucocorticoid–regulated kinase 1 (SGK1). Translation of HIF2α depends upon the activity of mTORC2 but not mTORC1, whereas HIF1α expression depends upon both mTORC1 and mTORC2. These findings are significant, as most conventional RCC possesses biallelic alterations in the VHL gene, resulting in the accumulation of both HIF1α and 2α. Despite overlapping effects on gene expression, HIF2α is more relevant to the development and progression of RCC because it preferentially activates VEGF, transforming growth factor-alpha (TGFα), the stem cell factor Oct4, and Cyclin D1. In preclinical models, HIF2α suppression prevented tumor formation in von hippel lindau (VHL) defective renal carcinoma cells. Importantly, mTORC2 activity is unaffected by rapamycin and its analogues, the mTOR inhibitors currently used in common practice. Consequently, HIF1α levels decrease, but HIF2α remains unaffected by rapamycin in preclinical RCC models.
PI3K/AKT/mTOR in RCC
One study examined 128 primary RCCs, 22 metastatic RCCs, and 24 nonneoplastic (normal) kidneys, and found the expression levels of p70S6K, phospho-mTOR (p-mTOR), and pAkt significantly higher in RCC than in normal kidneys, both by immunohistochemistry and protein levels. Similarly, another group found increased levels of phospho-S6 and/or p-mTOR in the majority of 29 clear cell RCC tumors by immunohistochemistry. A more recent report looking at 132 metastatic RCC samples, a subset with matched primary tumors, and an additional 10 sections from healthy kidney tissues found strong expression of various mTOR pathway proteins. The investigators reported significantly higher immunoreactivity scores for PI3K, p-mTOR, p-AKT, and p70S6 in metastatic lesions compared to nonneoplastic proximal tubular epithelial cells. Notably, there were almost no cases of PTEN gene deletion in this series. There was no significant correlation between primary tumors and metastatic samples in matched pairs, suggesting that hyperactivation of the pathway may contribute to the metastatic progress. Patients with shorter disease-free intervals showed significantly higher expression of PI3K ( P = .035). Similarly, a separate report in primary RCC correlated mTOR pathway activation with survival and poor pathologic prognostic features.
PI3K/AKT/mTOR in RCC
One study examined 128 primary RCCs, 22 metastatic RCCs, and 24 nonneoplastic (normal) kidneys, and found the expression levels of p70S6K, phospho-mTOR (p-mTOR), and pAkt significantly higher in RCC than in normal kidneys, both by immunohistochemistry and protein levels. Similarly, another group found increased levels of phospho-S6 and/or p-mTOR in the majority of 29 clear cell RCC tumors by immunohistochemistry. A more recent report looking at 132 metastatic RCC samples, a subset with matched primary tumors, and an additional 10 sections from healthy kidney tissues found strong expression of various mTOR pathway proteins. The investigators reported significantly higher immunoreactivity scores for PI3K, p-mTOR, p-AKT, and p70S6 in metastatic lesions compared to nonneoplastic proximal tubular epithelial cells. Notably, there were almost no cases of PTEN gene deletion in this series. There was no significant correlation between primary tumors and metastatic samples in matched pairs, suggesting that hyperactivation of the pathway may contribute to the metastatic progress. Patients with shorter disease-free intervals showed significantly higher expression of PI3K ( P = .035). Similarly, a separate report in primary RCC correlated mTOR pathway activation with survival and poor pathologic prognostic features.
Rapamycin and its analogues
Rapamycin (also termed sirolimus) was originally identified as a natural antifungal antibiotic isolated from the bacteria Streptomyces hygroscopicus in the 1970s and eventually led to the discovery of mTOR, the “mammalian target of rapamycin.” Due to its ability to potently inhibit T-cell function, rapamycin was initially mainly used as an immunosuppressant in recipients of solid organ transplantation, but subsequently was found to be an attractive candidate for application in oncology due to its antitumor activity, including preclinical models for RCC. Several analogues of rapamycin have been developed to improve solubility and bioavailability. These include temsirolimus and everolimus, and are termed “rapalogs.” They share the same mechanism of action and have been successfully applied in the treatment of various solid and hematologic malignancies. Rapamycin and its analogues do not directly inhibit the mTOR kinase. Instead, they bind with high affinity to the FK-binding protein 12 (FKBP-12), an abundant intracellular immunophilin. The resulting complex potently inhibits the kinase activity of mTORC1, but has no suppressive effects on mTORC2.
Temsirolimus
Temsirolimus (Torisel; Pfizer, New York, NY, USA) is a water-soluble prodrug of rapamycin with an added ester at the C43 position. It is rapidly metabolized to sirolimus through de-esterification; both are potent binders of FKBP-12, and each forms an inhibitory complex with subsequent suppression of mTORC1 activity.
Phase I and II Studies
Preclinical studies demonstrating temsirolimus activity in a variety of human cancers were fortified by promising results in phase 1 studies. Intermittent schedules abrogated immunosuppressive effects without significant loss in antitumor activity. Responses included patients with advanced RCC. This led to a dedicated phase II trial in advanced refractory RCC. With a primary endpoint of objective tumor response rate, 111 patients were randomized to 25, 75, or 250 mg of temsirolimus weekly by intravenous infusion. Patients were heavily pretreated (51% had received ≥2 prior immunotherapies) with extensive disease (83% had ≥2 sites of metastases). The objective response (OR) rate was 7% (1 complete response, 7 partial responses [PR]); 51% had stable disease (SD) for ≥24 weeks or an OR. Median time to progression (TTP) was 5.8 months for the entire group; 6.3, 6.7, and 5.2 for patients in the 25-, 75-, and 250-mg groups, respectively. Median overall survival (OS) was 15 months; 13.8, 11.0, and 17.5 for patients in the 25-,75-, and 250-mg groups, respectively. Maculopapular rash (76%) and mucositis (70%) were the most frequent treatment-related toxicities; the most common grade 3 or 4 adverse events (AEs) included hyperglycemia (17%), hypophosphatemia (13%), anemia (9%) and hypertriglyceridemia (6%). Importantly, overall response rates (ORR) and OS were comparable for all dose levels. Dose reductions and treatment discontinuations were more frequent at higher doses. A subgroup analysis by Memorial Sloan-Kettering Cancer Center (MSKCC) risk group, developed for patients treated with interferon (IFN)-α, demonstrated greater than twofold survival differences between good or intermediate versus poor-risk patients at each dose level. Compared with historical data for IFN-α, treatment benefit was most striking for the poor-risk population.
Phase III Data
The Global Advanced Renal Cell Carcinoma (ARCC) phase III trial, conducted between 2003 and 2005, compared temsirolimus to IFN-α, or the combination, in advanced RCC. Entry criteria allowed all histologic subtypes, but required participants to have at least three of six predictors of short survival ( Table 1 ). Patients were randomized to one of three arms: temsirolimus 25 mg intravenously (IV) once weekly, IFN-α 3 million units subcutaneously three times per week (escalated to 18 million units three times per week, if tolerated), or a combination of temsirolimus 15 mg IV weekly and IFN 3 million units(escalated to 6 million units three times per week). Efficacy at the second planned interim analysis of the intent-to-treat population revealed superior survival for temsirolimus over IFN-α but no improved survival for the combination over IFN-α alone. Median OS was 7.3, 10.9, and 8.4 months for IFN-α, temsirolimus, and the combination group, respectively. Progression-free survival (PFS) was significantly longer in patients receiving temsirolimus, with median PFS times of 1.9, 3.8, and 3.7 months for the IFN-α, temsirolimus, and the combination group, respectively ( P <.001). The proportion of patients with SD greater than or equal to 6 months or an OR was significantly greater for patients receiving temsirolimus alone (32.1%) or in combination (28.1%) than in the IFN-α group (15.5%; P <.001 and P = .002, respectively). In prespecified exploratory subgroup analyses, the superior survival benefit of temsirolimus was greatest for patients less than 65 years of age and for those with elevated lactate dehydrogenase. The most common all-grade toxicities ( Table 2 ) for the temsirolimus group were managed with supportive measures. Fewer grade 3 or 4 AEs were seen with temsirolimus alone than with IFN-α or the combination (67% vs 78% vs 87%, respectively; P = .02). Most dose reductions or delays were reported for the combination group. Based on this study, in May 2007, temsirolimus received FDA approval for the treatment of advanced RCC.
| ARCC Trial | RECORD 1 Trial | |||
|---|---|---|---|---|
| Number of Patients | 626 | 416 | — | |
| Histologies | All | Clear cell RCC component | — | |
| Prior Therapy | None | Sunitinib, sorafenib or both | — | |
| Clinical Risk | All with ≥3/6 poor-risk features: | |||
| LDH ≥ 1.5 × ULN decreased Hgb corrected Ca of ≥10 mg/dL time diagnosis to treatment initiation of <1 year KPS 60%–70% ≥2 metastatic sites | Favorable risk a | 29% | ||
| Intermediate risk a | 57% | |||
| Poor risk a | 15% | |||
| (well balanced between groups) | ||||
| Randomization Arms, Number of Patients | IFN: 207 Tem: 209 IFN/Tem: 210 | Everolimus: 277 Placebo: 139 | ||
| Primary endpoint | OS | PFS | ||
| Results | ||||
|---|---|---|---|---|
| Responses | 1st-Line Tem | 1st-Line IFN | 2nd-Line Everolimus | 2nd-Line Placebo |
| mPFS (mo) | 3.8 (CI 3.6–5.2) b | 1.9 (CI 1.9–2.2) b | 4.9 (CI 4.0–5.5) c | 1.9 (CI 1.8–1.9) c |
| mOS (mo) | 10.9 (CI 8.6–12.7) d | 7.3 (CI 6.1–8.8) d | 14.8 e | 14.4 f |
| Response | ORR: 9% (CI 4.8–12.4) Benefit g : 32% (CI 25.7–38.4) | ORR: 5% (CI 1.9–7.8) Benefit g : 16% (CI 10.5–20.4) | Best response: PR: 2% SD: 67% (CI not reported) | Best response: PR: 0% SD: 32% (CI not reported) |
a Risk stratification per MSKCC clinical risk score.
c HR: 0.33; CI 0.25–0.43; P <.001.
d HR: 0.73; CI 0.58–0.92; P = .008.
e HR: 0.87; CI 0.65–1.15; P = .16.
f Rank preserving structural failure time approach estimated true mOS for placebo group to be 10.0 months.
| Adverse Events | 1st-Line Temsirolimus in Poor-risk Patients | 2nd-Line Everolimus after Prior VEGF TKI | ||
|---|---|---|---|---|
| All Grades (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) | |
| Asthenia | 51 | 11 | 33 | 3–4 |
| Fatigue | NR | 31 | 5 | |
| Rash | 47 | 4 | 29 | 1 |
| Nausea | 37 | 2 | 26 | 1 |
| Anorexia | 32 | 3 | 25 | 1 |
| Pain | 28 | 5 | NR | |
| Dyspnea | 28 | 9 | 24 | 7 |
| Infection | 27 | 5 | 37 | 7 |
| Diarrhea | 27 | 1 | 30 | 1 |
| Constipation | 20 | 0 | NR | |
| Peripheral edema | 27 | 2 | 24 | <1 |
| Cough | 26 | 1 | 30 | <1 |
| Dyspnea | NR | 24 | 7 | |
| Pneumonitis a | NR | 14 a | 4 a | |
| Fever | 24 | 1 | 20 | <1 |
| Abdominal Pain | 21 | 4 | NR | |
| Stomatitis | 20 | 1 | 44 | 4–5 |
| Vomiting | 19 | 2 | 20 | 2 |
| Headache | 15 | 1 | 19 | 0–2 |
| Epistaxis | NR | 18 | 0 | |
| Pruritus | NR | 14 | <1 | |
| Dysgeusia | NR | 10 | 0 | |
| Laboratory Abnormalities | ||||
| Anemia | 45 | 20 | 92 | 13 |
| Thrombocytopenia | 14 | 1 | 23 | 1 |
| Lymphopenia | NR | 51 | 16 | |
| Neutropenia | 7 | 3 | 14 | <1 |
| Increased Creatinine | 14 | 3 | 50 | 1 |
| Hyperlipidemia | 27 | 3 | 73 | <1 |
| Hypercholesterolemia | 24 | 1 | 77 | 4 |
| Hyperglycemia | 26 | 11 | 57 | 15–16 |
| Increased AST | 8 | 1 | 25 | 0–2 |
| Hypophosphatemia | NR | 37 | 6 | |
a Includes interstitial lung disease, lung infiltration, pneumonitis, pulmonary alveolar hemorrhage, alveolitis, and pulmonary toxicity.
This study included patients with both conventional and non-clear cell histologies. Patients with histologies other than clear cell RCC accounted for 17% and 18% in the temsirolimus and interferon group, respectively. An unplanned secondary analysis for this patient subset was undertaken and suggested superior median OS and PFS for temsirolimus versus IFN-α with a hazard ratio (HR) of 0.49 (95% CI, 0.29–0.85) and 0.38 (95% CI, 0.23–0.62), respectively. Whereas median OS was shorter in non-clear cell histologies compared with conventional RCC, the benefit of temsirolimus appeared more pronounced with non-clear cell or indeterminate primary cell types. This may be because IFN has fewer efficacies in this group.
Temsirolimus in Second-line Setting
A randomized phase III trial has completed accrual ( clinicaltrials.gov ; NCT00474786), comparing temsirolimus with sorafenib in advanced RCC of any histology after progression on sunitinib, but no prospective second-line data have been reported thus far. The largest retrospective series reported outcome for 87 patients treated at North American centers through the Torisel Compassionate Use Program. The majority of patients had been pretreated with sunitinib (85%) or sorafenib (51%), 63% had pure clear cell histology. The study population overall had unfavorable clinical features, mostly with either intermediate (53%) or poor (36%) MSKCC risk status ; 13% of patients had an Eastern Cooperative Oncology Group (ECOG) performance status of three. The reported ORR was 5% (all PR); best response of SD was 65%. The median TTP was 3.9; median OS was 11.2 months. Benefit was seen for both clear cell and non-clear cell histologies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree