mTOR Inhibitors
Adam Faletsky
Shaiba Sandhu
Herve Y. Sroussi
Lauren M. Guggina
The mammalian target of rapamycin (mTOR) is an important factor in cell growth and survival. mTOR is a protein kinase with two well-known subunits: mTOR complexes 1 and 2 (mTORC1 and mTORC2). These subunits drive important cellular processes such as protein synthesis and inhibition of autophagy. Downstream targets of mTOR are believed to affect cell growth, and overactivation can promote tumorigenesis and metastasis. Common mTOR inhibitors (mTORi) include sirolimus, everolimus, temsirolimus, and ridaforolimus and are used to treat cancers such as renal cell carcinoma, pancreatic neuroendocrine tumors, and advanced breast cancer. The use of mTORi is broadening, with many ongoing clinical trials evaluating their use as monotherapies and in combination regimens.1
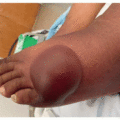
Oral toxicities: mTOR inhibitor-associated stomatitis (mIAS) is a dose-dependent oral toxicity that has been documented in up to 60% of patients treated with this targeted therapy.2,3 It presents as painful, well-demarcated round/ovoid single or multiple oral ulcers covered with yellowish pseudo membrane, commonly involving nonkeratinized mucosa (Figure 32.1).2,3 Although these ulcers usually clinically mimic recurrent aphthous stomatitis, mIAS regresses completely upon therapy withdrawal, without recurrence. Stomatitis associated with mTORi also differs from chemotherapy-induced mucositis, in which ulcers tend to be more diffuse.2,3,4 Of note, medication-related osteonecrosis of the jaw (MRONJ) has been infrequently reported with everolimus, likely due to the antiangiogenic effects and its impact upon bone turnover.5
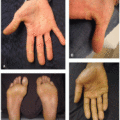
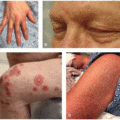
Acneiform eruptions: Common cutaneous events secondary to mTOR inhibition include acne-like lesions (46%, Figure 32.2), scalp folliculitis (26%), and hidradenitis suppurativa (12%). Acne-like lesions and folliculitis reported with sirolimus used for organ transplantation are typically moderate in severity, male predominant, and are not dose related.6 Acneiform lesions are similar to those that appear with anti-epidermal growth factor receptor therapy (EGFR),3 though typically more mild, and appear as papules or pustules that lack comedones and nodulocystic lesions.
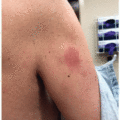
Exanthems: Inflammatory eruptions present in 27% to 49% of patients taking mTORi and are described as morbilliform/maculopapular or eczematous (Figure 32.3).4,7 Patients commonly present with xerosis and pruritus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree