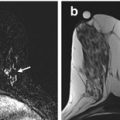
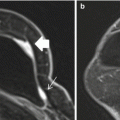
Fig. 10.1
Axial and sagittal T1 post contrast images demonstrate an irregularly shaped and irregularly marginated mass with heterogeneous enhancement (arrows). Biopsy demonstrated ER/PR + HER-2- IDC
10.3.2 Her2 Enriched
Although Her 2 positive tumors are not a perfect correlate for the HER2 enriched subtype, 60 % of HER-2 positive tumors are HER-2 enriched tumors. MRI features of HER2-positive tumors have been documented. HER-2 positive tumors have been reported to present as masses with microcalcifications [4]. Calcifications have been noted in as many as 78 % of HER2-positive tumors [4]. HER-2 cancers are often associated with DCIS, more than other breast cancer subtypes. Youk and colleagues reported MR characteristics of 94 HER2-positive cancers: mass enhancement was noted in 90 %; 47 % of masses were round or oval and 41 % were lobulated. Margins were spiculated (51 %) and irregular (48 %). Heterogeneous enhancement was most commonly seen (79 %) and washout was the most common kinetic pattern (90 %) Her-2-positive cancers demonstrate non-mass enhancement more often than the other subtypes [4] (Fig. 10.2).
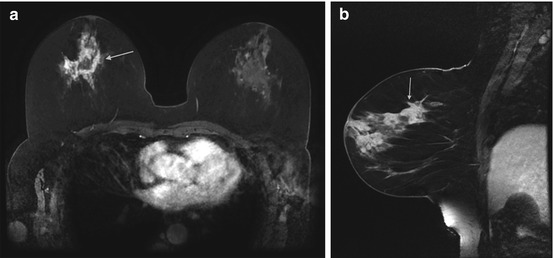

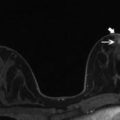
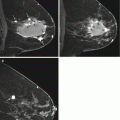
Fig. 10.2
Axial and Sagittal T1 post contrast images demonstrate an irregular mass with associated nonmass enhancement (arrow). Biopsy revealed HER2+ IDC
10.3.3 Triple Negative/Basal
MRI characteristics reflect histology in triple negative cancers/basal-like breast cancers [5–6]. Triple negative breast cancer (TNBC) refers to invasive cancers that lack estrogen receptors (ER negative), progesterone receptors (PR negative), and are human epidermal growth factor receptor negative (HER2 negative). The majority of TNBCs are basal like. TNBCs are associated with the BRCA1 mutation and early metastatic disease [6]. Clinically, this denotes a poor prognosis because tumors that are ER/PR negative do not respond to hormonal therapy. Also, targeted therapy with monoclonal antibodies against HER 2 will not work in tumors that are HER2 negative. TNBCs are typically high-grade [5]. Histologically, triple negative breast cancers are associated with the presence of a central scar, tumor necrosis, the presence of spindle cells or squamous metaplasia, high total mitotic count, and high nuclear-cytoplasmic ratio [5].
TNBCs often appear as circumscribed round or oval masses and are less likely to show distortion or calcifications [7]. Ultrasound frequently demonstrates a solid hypoechoic or mixed echogenicity oval, round or irregular mass with circumscribed or indistinct margins [5, 8]. TNBCs are generally seen as mass lesions on MRI with very few appearing as non-mass enhancement (NME) (Fig. 10.3). Masses may be round, oval or irregular with circumscribed, irregular or spiculated margins. Differentiation from benign masses can be difficult, as many features such as oval, circumscribed masses, persistent enhancement, and high T2 signal are seen both in benign lesions and TNBCs, although the high T2 signal in a TNBCs is often due to necrosis (Fig. 10.3). Rim-enhancement and internal, enhancing septations should increase suspicion if seen [7]. TNBCs are more likely than other cancers to have persistent enhancement kinetics [7].
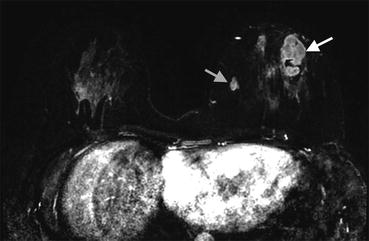

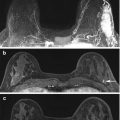
Fig. 10.3
Axial T1-weighted post contrast subtraction image demonstrates an irregularly shaped circumscribed mass with heterogeneous enhancement (white arrow). A smaller similar mass is noted medially (gray arrow). Biopsy of the large and small masses yielded triple negative cancer
10.4 MRI Tools for Detecting Invasive Cancer
10.4.1 MR-BI-RADS and Descriptors of Invasive Carcinomas
The MR-BI-RADS lexicon identifies specific morphologic and kinetic characteristics for Enhancing lesions and is discussed in Chap. 2, so will only briefly be addressed here. The combination of morphologic and kinetic features has a reported sensitivity of 90 % and a specificity of 72 % for detecting malignancy [2].
10.4.1.1 Morphologic Features of Invasive Disease
Invasive cancer may present as a mass, NME or a focus on MRI. A 2007 study reviewed MRI biopsy results found the probability of malignancy to be 34 % for masses, 27 % for NME, and 19 % for foci [9]. Certain lesion characteristics should raise suspicion for malignancy and invasive disease, in particular masses with irregular and spiculated margins [10, 11]. A 2006 study documenting the characteristics of 171 masses on MRI demonstrated the most frequent morphological finding in malignant lesions was heterogeneous internal enhancement (96 % of malignancies demonstrated in the delayed phase and 90 % in the early phase). Features with the highest positive predictive value for carcinoma were spiculated margin (100 %), delayed central enhancement (100 %), enhancing internal septations in the delayed phase (97 %), and irregular shape (97 %). Of the masses studied 25 % of smooth round or oval masses were malignant, 85 % of irregularly-shaped or marginated masses were malignant and 100 % of spiculated masses were malignant. Smooth margins were the most frequent finding in benign lesions (80 %) [11]. Regarding masses, a 2012 study of 969 patients demonstrated the highest PPV was found with irregular margins (PPV, 0.196) and spiculated margins, (PPV, 0.333). The lowest PPV was found with smooth margins (PPV, 0.052). Masses with marked internal enhancement were most likely to represent cancer (PPV, 0.231). Both plateau (PPV 0.152) and washout (PPV, 0.178) were associated with cancer [12].
10.4.1.2 Kinetic Features of Invasive Disease
Tumor enhancement may be plotted on a time-signal intensity curve. The initial contrast enhancement and the delayed contrast enhancement characteristics are calculated. These kinetic curves have become a standard component of the breast MRI exam and allow for better prediction of malignancy. Due to angiogenesis, malignancies demonstrate rapid wash-in and wash-out of the contrast agent. This is known as a type 3 time-signal intensity curve. This type 3 pattern is the most concerning curve type. However, benign entities (such as lymph nodes) may demonstrate this washout curve. In addition, a persistent or plateau curve may be seen in both benign and malignant lesions [10, 13]. A 2010 study comprised of 120 malignancies, both in situ and invasive cancers, demonstrated persistent enhancement in 10 % of masses, plateau enhancement in 48.6 %, and washout in 41 % of masses [13]. Enhancement patterns of NME were less specific for malignancy [13].
10.4.2 Evolving MRI Techniques for Imaging Invasive Disease
10.4.2.1 Diffusion Weighted Imaging
New functional MRI tools may improve the sensitivity of MRI for detecting invasive disease. Diffusion weighted imaging (DWI) is one such technology (Fig. 10.4) and is discussed in detail in Chap. 15. Malignant breast tumors have high cellularity and often demonstrate restricted water diffusion (high signal intensity) and lower apparent diffusion coefficient (Fig. 10.4). Studies demonstrate an increase in specificity compared to contrast-enhanced MRI alone [14]. A 2010 study of 84 breast lesions demonstrated 97.9 % sensitivity and 75.7 % specificity of DWI. The addition of DWI to standard MRI may be particularly helpful in increasing MRI specificity for malignancy, with one study demonstrating a 13.5 % increase in specificity [15].
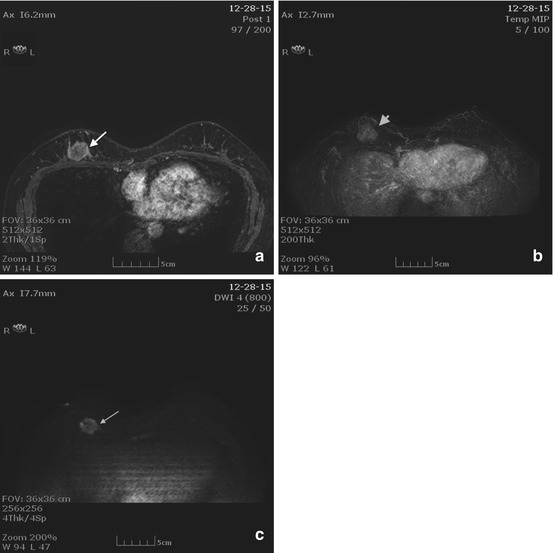

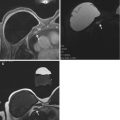
Fig. 10.4
(a) Axial T1 post contrast image demonstrates a round rim enhancing mass with an irregular border (arrow). (b) MIP image demonstrates enhancement within the mass (arrowhead). (c) DWI image demonstrates bright signal within the mass indicating restricted diffusion (arrow)
10.4.2.2 MR Proton Spectroscopy
Proton MR spectroscopy (1H-MRS) is discussed in detail in Chap. 15. Spectroscopy may be used to help characterize lesions. The choline concentrations in tumors may be associated with increased membrane synthesis by replicating cells and therefore with biologic aggressiveness [16]. Increased total choline-containing compound has been associated with overexpression of the HER-2neu gene [17] and with an aggressive breast cancer phenotype [17, 18]. Studies show that tCho detection rate is higher in invasive cancer compared to DCIS, possibly associated with more aggressive behavior and/or faster cell replication.
Choline kinase overexpression has been found to be significantly associated with high histologic grade and ER-negative status [16]. These associations may be due to increased cell proliferation. ER is considered a favorable prognostic indicator in breast tumors as ER-positive tumors are more likely to be well differentiated and less aggressive. Patients with ER-positive tumors have more therapeutic options, such as ER blockers or aromatase inhibitors, than do patients with ER-negative tumors. A study of ER status and MR spectroscopic features found that the total choline-containing compound detection rate was higher in ER-negative patients [16].
HER-2/neu is associated with an aggressive tumor phenotype and reduced survival rate. The intracellular domain of HER-2 neu has tyrosine kinase activity that regulates cell growth and proliferation [19]. HER-2 neu is overexpressed in 20–25 % of invasive breast cancers and has been associated with more aggressive tumors, early relapse, and shorter survival [20]. The choline detection rate has been found to be higher in HER-2 neu- positive than in HER-2 neu-negative tumors. Additionally, triple-negative tumors showed significantly higher signal-to-noise ratio (SNR) than did non–triple-negative tumors [20]. Shin and colleagues found that on MRS, IDCs were consistently positive for choline whereas DCIS and IDC with an extensive intraductal component (EIC) were likely negative [18]. SNR was significantly higher in tumors of high histologic grade than lower histologic grade [18]. In summary, proton MRS may be an imaging biomarker for malignancy.
10.5 Radiologic-Pathologic Correlation and Invasive Disease
10.5.1 Specific Tumor Types and MRI Appearance
Traditional pathologic classification divides invasive disease into two major subtypes: invasive ductal carcinoma and invasive lobular carcinoma. Invasive ductal carcinoma not otherwise specified (IDC-NOS) is the most common. The remaining ductal cancers are further subdivided into unusual ductal carcinomas including papillary, micropapillary, mucinous, tubular, and adenoid cystic. Stromal malignancies including metaplastic carcinomas, phyllodes, and sarcomas are rarely encountered. MRI appearance varies according to histologic type; however, tremendous overlap is present.
10.5.2 Invasive Ductal Carcinoma NOS
IDC NOS comprises 85 % of breast cancers. The presence of glandular differentiation and intercellular cohesion defines ductal differentiation. However, most ductal carcinomas consist of invasive tubules and glands and have no specific type designation [21]. These tumors often elicit a scirrhous reaction, resulting in the irregular border seen on MRI. However, high-grade ductal carcinomas may grow so rapidly that there is no time for a scirrhous reaction, resulting in circumscribed borders. This circumscribed appearance is more common in BRCA related carcinomas [21].
Common MRI findings are an irregularly-shaped mass with heterogeneous enhancement and irregular or spiculated margins (Fig. 10.5). Also, peripheral or rim enhancement can be seen. Although IDC-NOS is typically not bright on T2 -weighted images, some IDC-NOS demonstrate areas of T2 hyperintensity secondary to necrosis [22]. The margins of lesions are often best characterized on nonfat saturated T1- weighted images. Surrounding breast tissue must be carefully evaluated, as small satellite masses may be present. Satellite lesions are masses with similar enhancement characteristics as the primary cancer, but are smaller, and usually in close proximity. Contrast enhancement characteristics may vary but the most common pattern is rapid wash-in and rapid wash-out (type 3 curve) [22].
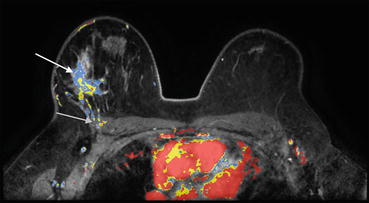

Fig. 10.5
Axial post contrast color image shows an irregular mass with heterogeneous enhancement (white arrow). Note the loss of a fat plane between the mass and the pectoralis muscle, as well as enhancement of the pectoralis (gray arrow). Biopsy revealed IDC NOS
10.5.3 Papillary Carcinoma
10.5.3.1 Histology and Presentation
Papillary carcinoma is a rare variant of invasive ductal carcinoma accounting for less than 2 % of carcinomas and is most commonly found in postmenopausal women [23, 24]. Histologically, the epithelium proliferates into villous-like projections that eventually fill the lumen [23]. Papillary carcinoma is differentiated from a papilloma by the malignant appearing epithelial cells and an absent myoepithelial layer. Papillary carcinomas are subdivided into solid, intracystic without invasion, intracystic with a focus of invasion, and invasive papillary carcinoma [24]. This carcinoma may arise in the central ducts and is located in the retroareolar region in about 50 % of patients [24]. Bloody nipple discharge is present in 22–34 % of patients [23]. Patients with papillary carcinoma often have a better prognosis than patients with IDC-NOS. Axillary lymph nodes are involved less often in patients with papillary carcinoma than in patients with other types of ductal carcinoma [23].
10.5.3.2 Imaging
Papillary carcinomas are frequently round, oval, and circumscribed on mammography [23]. This round appearance is due to their cystic component [25]. Papillary cancers do not produce a fibrotic reaction and generally do not show spiculation by mammography. Ultrasound features are a solid hypoechoic or mixed solid and cystic mass with vascularity [23]. Differentiation from benign papillary lesions can be difficult. MRI features of papillary carcinomas are an irregular or round, enhancing mass, often near the nipple. Papillary carcinomas may be bright on both T1 and T2 images. [25]. Intracystic papillary carcinoma will have hyperintensity on T2-weighted images (Fig. 10.6). Enhancement curves can vary from type 1 to type 3. MRI may be helpful in delineating multiple papillary masses [25].
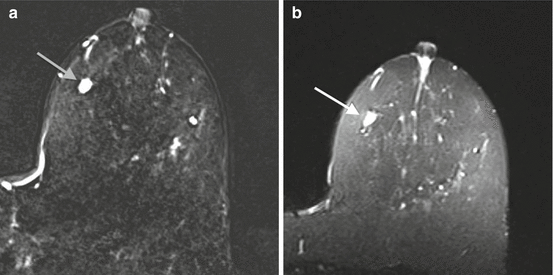

Fig. 10.6
(a) Axial T1 post contrast subtraction image demonstrating and oval circumscribed enhancing mass (gray arrow). (b) Axial T2 image demonstrating increased signal intensity (white arrow). Biopsy revealed papillary carcinoma
10.5.4 Invasive Micropapillary Carcinoma
10.5.4.1 Histology and Presentation
Invasive micropapillary carcinoma (IMPCa) is a histologic pattern of breast cancer characterized by small, tightly cohesive groups of neoplastic cells disposed within well-delineated clear spaces resembling lymphatic vessels [26]. Micropapillary carcinomas of the breast are described pathologically as having numerous small pseudo-papillary clusters of cells without fibrovascular cores and clusters surrounded by clear spaces [23, 27]. Micropapillary carcinomas account for less than 2 % of breast cancers [23]. They have a worse overall prognosis than IDC-NOS [23]. Various studies report varying percentages of metastatic disease to axillary lymph nodes from 64 to 90 % [27, 28].
10.5.4.2 Imaging
Mammographic appearances include masses displaying a lobulated or irregular shape with spiculated or indistinct margins. Architectural distortion may also be present [23]. When calcifications are associated, they are typically fine pleormorphic or linear branching. Ultrasound features include a hypoechoic or mixed echogenicity mass with irregular shape and spiculated, microlobulated, or indistinct margins. MRI findings include masses displaying an oval/round or irregular shape with irregular or spiculated margins [29] (Fig. 10.7). Initial rapid enhancement with washout or plateau kinetics in the delayed phase may be observed. Internal enhancement may be homogeneous or heterogeneous [29]. NME has also been reported. Careful attention should be paid to lymph nodes as invasive micropapillary carcinoma has a predilection for lymph node involvement [29].
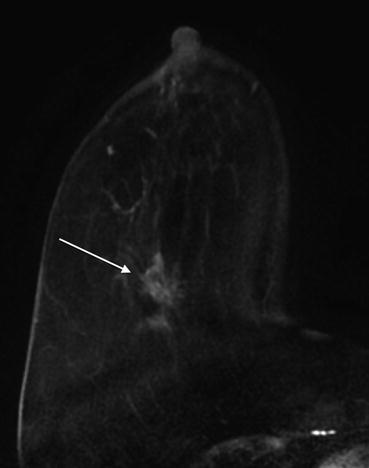

Fig. 10.7
Axial T1 subtraction image demonstrates an enhancing spiculated mass with spiculated borders (arrow). Biopsy revealed invasive micropapillary carcinoma
10.5.5 Medullary Carcinoma
10.5.5.1 Histology and Presentation
Medullary carcinoma of the breast is rare, accounting for less than 5 % of breast cancers [30]. It is defined by the World Health Organization (WHO) classification of breast tumors as “a well-circumscribed carcinoma composed of poorly differentiated cells arranged in large sheets, with no glandular structure, scant stroma, and a prominent lymphoplasmacytic infiltrate” [30]. It is most commonly seen in women in their late 40s and early 50s and has a more favorable prognosis than IDC-NOS [30]. A higher incidence of medullary carcinoma is noted in patients with BRCA1 mutation. A series of 1490 patients managed with breast-conservation therapy that consisted of lumpectomy and radiation therapy at Yale University included 46 cases of medullary carcinoma. The 10-year distant relapse-free survival in the medullary cohort was significantly better than in the control group of IDC NOS (94.9 % vs. 77.5 %, p = 0.028) [30].
10.5.5.2 Imaging
Mammographic appearance is a dense oval or round mass with circumscribed or microlobulated margins. Ultrasound appearance may be confused with a fibroadenoma [30]. Often medullary carcinomas are circumscribed, hypoechoic, parallel masses with varying degrees of through transmission [30]. The MRI appearance has been reported as isointense on T1-weighted and isointense or slightly hyperintense on fat-saturated T2-weighted images. Medullary carcinomas have oval or round shapes and smooth margins upon contrast enhancement. Heterogeneous enhancement with delayed, peripheral enhancement on late-phase contrast MRI has been reported [30]. Rapid wash-in and rapid wash-out or plateau enhancement is often seen. The peripheral rim enhancement correlates with a peripheral compressed fibrous tissue with prominent lymphocytic infiltration noted at pathology [30] (Fig. 10.8).
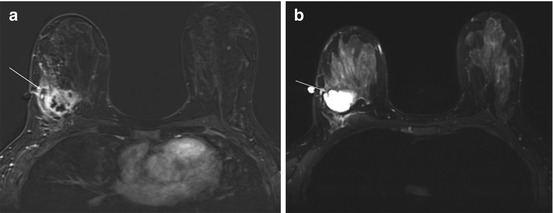

Fig. 10.8
(a) Axial T1 post contrast subtraction image demonstrates a mass with an irregular border and rim enhancement characteristic of medullary carcinomas (white arrow). (b) T2 image demonstrates hyperintense signal within the mass (gray arrow). Biopsy revealed medullary carcinoma
10.5.6 Mucinous Carcinoma
10.5.6.1 Histology and Presentation
Mucinous carcinoma, also known as colloid, mucous, or mucoid carcinoma of the breast is a well-differentiated type of invasive adenocarcinoma characterized by large amount of extracellular epithelial mucus. Mucinous carcinoma constitutes 1–7 % of breast carcinomas. Two subtypes of mucinous carcinoma may be differentiated histologically: pure and mixed. The pure type typically has indolent growth, while the mixed type has variable biological behavior, often similar to IDC-NOS. The pure type typically demonstrates a lower histological grade (well-differentiated tumors), higher hormone receptor expression, lower incidence of adverse oncogenes, lower rate of axillary lymph node involvement at diagnosis, and longer disease-free survival [31].
10.5.6.2 Imaging
Mucinous carcinoma may present as circumscribed lesions. A multimodality approach is helpful to reach appropriate diagnosis as well as to differentiate between the two histological types of the neoplasm. Typically, a mucinous carcinoma presents as an oval or round mass with circumscribed margins. At mammography, the pure type correlates with circumscribed or microlobulated margins while the mixed type presents with more indistinct or spiculated contours secondary to a higher degree of fibrosis and peripheral desmoplasia, similar to a IDC-NOS. Microcalcifications are uncommon and may be associated with peripheral component of DCIS [32]. Sonographically, mucinous carcinomas are often heterogeneous in echogenicity and may have mixed solid and cystic components. Posterior acoustic enhancement is common [33].
Mucinous carcinoma on MRI typically has a lobular shape, homogeneous and markedly high signal intensity on T2-weighted images, and a persistent enhancement pattern on dynamic MR images (with rim-like peripheral or heterogeneous internal enhancement). Thus, it has MR imaging features of both benignity and malignancy (Fig. 10.9). The combination of MR imaging features is useful for preoperative diagnosis of the tumor [34]. High signal intensity on T2-weighted images is seen in a pure mucinous carcinoma because the entire tumor is filled by mucin. In mixed mucinous carcinomas, the solid component is identifiable by its relative signal hypointensity on the T2-weighted images [35].
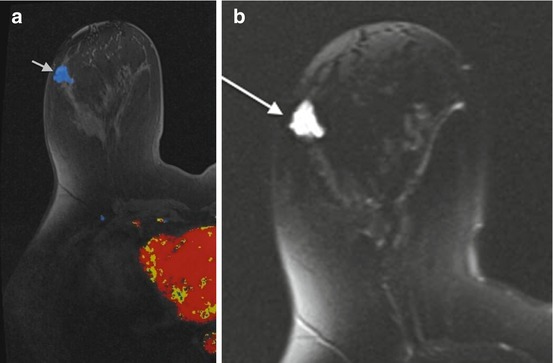

Fig. 10.9




(a) Axial T1 post contrast color image demonstrates an irregular mass with plateau (Type II) enhancement (gray arrow). (b) T2 image demonstrates high signal intensity (white arrow). Biopsy revealed mucinous carcinoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree