TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
Carcinoma in situ
Tis (DCIS)
Ductal carcinoma in situ
Tis (LCIS)
Lobular carcinoma in situ
Tis (Paget’s)
Paget’s disease of the nipple not associated with invasive carcinoma or carcinoma in situ (DCIS and/or LCIS) in the underlying breast parenchyma
T1
Tumor ≤20 mm in greatest dimension
T1mi
Tumor ≤1 mm in greatest dimension
T1a
Tumor >1 mm but ≤5 mm in greatest dimension
T1b
Tumor >5 mm but ≤10 mm in greatest dimension
T1c
Tumor >10 mm but ≤20 mm in greatest dimension
T2
Tumor >20 mm but ≤50 mm in greatest dimension
T3
Tumor >50 mm in greatest dimension
T4
Tumor of any size with direct extension to the chest wall and/or to the skin (ulceration or skin nodules)
Note: Invasion of the dermis alone does not qualify as T4
T4a
Extension to the chest wall, not including only pectoralis muscle adherence/invasion
T4b
Ulceration and/or ipsilateral satellite nodules and/or edema (including peau d’orange) of the skin, which do not meet the criteria for inflammatory carcinoma
T4c
Both T4a and T4b
T4d
Inflammatory carcinoma
Table 4.2
The American Joint Committee on Cancer staging system: breast regional lymph nodes (N)
Clinical | |
|---|---|
NX | Regional lymph nodes cannot be assessed (e.g. previously removed) |
N0 | No regional lymph node metastases |
N1 | Metastases to movable ipsilateral level I, II axillary lymph node(s) |
N2 | Metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted; or in clinically detecteda ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastases |
N2a | Metastases in ipsilateral level I, II axillary lymph nodes fixed to one another (matted) or to other structures |
N2b | Metastases only in clinically detecteda ipsilateral internal mammary nodes and in the absence of clinically evident level I, II axillary lymph node metastases |
N3 | Metastases in ipsilateral infraclavicular (level III axillary) lymph node(s) with or without level I, II axillary lymph node involvement; Or in clinically detecteda ipsilateral internal mammary lymph node(s) with clinically evident level I, II axillary lymph node metastases; Or metastases in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement |
N3a | Metastases in ipsilateral infraclavicular lymph node(s) |
N3b | Metastases in ipsilateral internal mammary lymph node(s) and axillary lymph node(s) |
N3c | Metastases in ipsilateral supraclavicular lymph node(s) |
Table 4.3
The American Joint Committee on Cancer staging system: breast distant metastases (M)
Mo | No clinical or radiographic evidence of distant metastases |
cM0(i+) | No clinical or radiographic evidence of distant metastases, but deposits of molecularly or microscopically detected tumor cells in circulating blood, bone marrow, or other nonregional nodal tissue that are no larger than 0.2 mm in a patient without symptoms or signs of metastases |
M1 | Distant detectable metastases as determined by classic clinical and radiographic means and/or histologically proven larger than 0.2 mm |
Table 4.4
The American Joint Committee on Cancer staging system: breast anatomic stage/prognostic groups
Stage | Tumor | Node | Metastasis |
|---|---|---|---|
0 | Tis | N0 | M0 |
IA | T1a | N0 | M0 |
IB | T0 T1a | N1mi N1mi | M0 M0 |
IIA | T0 T1a T2 | N1b N1b N0 | M0 M0 M0 |
IIB | T2 T3 | N1 N0 | M0 M0 |
IIIA | T0 T1a T2 T3 T3 | N2 N2 N2 N1 N2 | M0 M0 M0 M0 M0 |
IIIB | T4 T4 T4 | N0 N1 N2 | M0 M0 M0 |
IIIC | Any T | N3 | M0 |
IV | Any T | Any N | M1 |
Breast MRI is the most sensitive and accurate imaging modality for local-regional staging of breast cancer [2–7]. It is superior to clinical examination, mammography and ultrasound, alone or combined, in delineation of the size and extent of tumor, additional sites of disease, pectoralis muscle and chest wall invasion, nipple and skin involvement, as well as lymph node metastasis. The ability of MRI to assess the size and extent of the index tumor and to identify additional, otherwise occult disease of the index and contralateral breasts has added sensitivity and complexity to clinical staging and surgical planning.
4.2 Size and Extent of Index Tumor
All published studies show that breast MRI is the most accurate imaging tool for evaluation of the size and extent of breast tumor [2–7]. Lesion size as determined by MRI correlates best with the pathologic size assessment among all imaging modalities (Fig. 4.1), although overestimation and underestimation do occur. MRI may overestimate tumor size (by greater than 5 mm) in up to 35 % of cases and underestimate size in 13 % of cases [5, 6]. The causes of over- or under-estimation have yet to be defined. Some studies suggest that MRI tumor size correlates better with pathologic measurement with high-grade invasive tumor and high-grade ductal carcinoma in-situ (DCIS), and tends to underestimate size in low-grade tumors [8, 9]. However, a recent report showed high-grade tumor and DCIS to be the strongest negative factors resulting in overestimation of tumor size on MRI [10]. There is a greater tendency for tumor size overestimation when tumors are larger than 2 cm in size [6, 10]. The MRI sequence on which the tumors are measured may also be a factor. A recent report suggests that index tumor size is best measured on T2 weighted images, whereas the whole extent of disease is best estimated on early-subtracted dynamic contrast enhanced T1 weighted images [11].
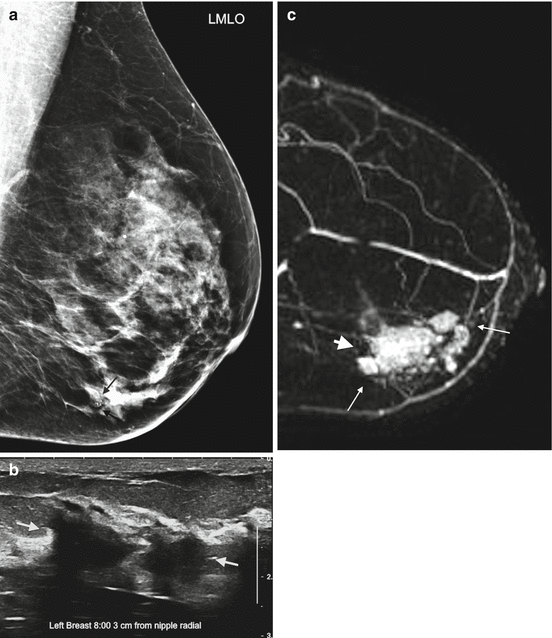

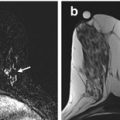
Fig. 4.1
Clinical stage IIA, T2N0M0 tumor in a 52-year-old with a palpable mass in the left breast and discordant tumor size between breast examination, mammography, and ultrasound. (a) Left mediolateral oblique view (MLO) mammogram reveals a small group of microcalcifications (black arrow). Biopsy revealed invasive lobular carcinoma. (b) Ultrasound of the left breast at the biopsy site shows two adjacent irregular hypoechoic masses, measuring 2.7 × 1.5 cm in aggregate. (c) Sagittal post contrast T1-weighted maximal-intensity projection (MIP) MR image reveals an irregular enhancing mass, 3.7 × 2.5 × 2.0 cm in size (between arrows). Arrowhead denotes focal susceptibility artifact caused by a tissue marker at the site of microcalcifications. Histopathology confirmed the large tumor size
MRI is more accurate than mammography or ultrasound for detection of an intraductal component of an invasive cancer (Figs 4.2 and 4.3). However, it may overestimate this finding in 11–28 % and underestimate it in 17–28 % of cases [12–14]. Overestimation may be due to enhancement of normal glandular tissue, other coexisting benign entities, or lymphovascular invasion [15]. Since extensive intraductal component (EIC) is a contributing factor for positive surgical margins at breast conserving surgery, preoperative delineation of the extent of EIC is essential.
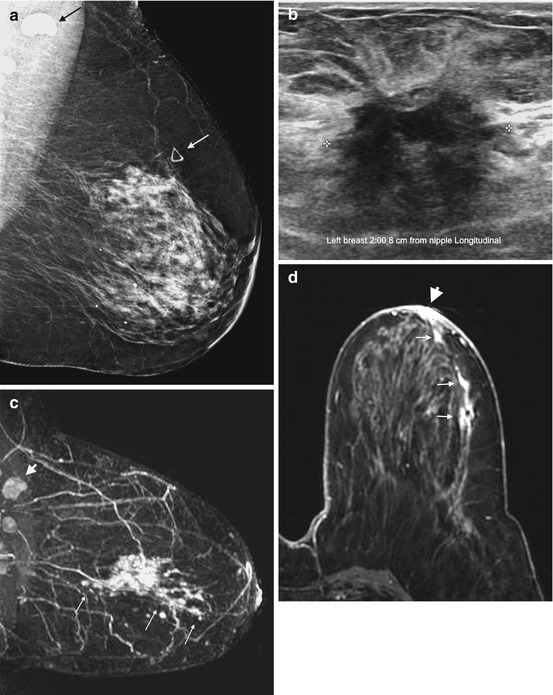
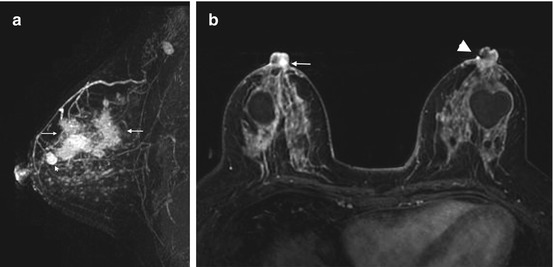

Fig. 4.2
Clinical stage IIIA, T3N1M0 tumor in a 72-year-old with extensive intraductal component (EIC) and unsuspected nipple involvement. (a) Left MLO view mammogram shows heterogeneously dense breast tissue with a triangular marker (white arrow) indicating a palpable mass. The mass is not visible on mammography. An abnormal high-density axillary lymph node is visible (black arrow). (b) Ultrasound of the palpable mass reveals a 2.7 cm irregular mass. Ultrasound guided biopsy confirmed invasive ductal carcinoma. Fine needle aspiration of the suspicious lymph node was positive for metastasis. (c) Sagittal post contrast T1 MIP MR image demonstrates an irregular enhancing mass corresponding to the known invasive cancer, with nonmass enhancement (long arrows) extending from the mass both anteriorly and posteriorly, consistent with EIC. The maximal anteroposterior extent of the tumor is 12 cm. Note the metastatic node with loss of fatty hilum (arrowhead). (d) Axial post contrast fat-saturated T1 MR image reveals nonmass enhancement in a ductal distribution (short arrows) extending to the nipple, with enhancement of the nipple-areolar complex (NAC) (arrowhead) consistent with tumor invasion

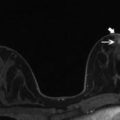
Fig. 4.3
Clinical stage IIIA, T3N1M0 tumor in a 42-year-old woman with multicentric right breast cancer, EIC, and nipple involvement. (a) Sagittal post contrast subtraction T1 MIP MR image of the right breast shows a small known invasive tumor (arrowhead) and extensive nonmass enhancement consistent with EIC, involving the upper outer and upper inner quadrants (between arrows). There is a metastatic lymph node in the axilla. (b) Bilateral axial post contrast fat-saturated T1 MR image demonstrates nodular enhancement in the right nipple (arrow), compared to non-enhancement of the left nipple. The focal signal abnormality in the left nipple (arrowhead) is an artifact
Contrary to early reports, MRI has been shown to be more sensitive in detection of DCIS than mammography and ultrasound (Fig. 4.4). This is largely attributable to a greater emphasis on high spatial resolution over high temporal resolution in MRI technique [16]. Reported MRI sensitivity for DCIS in the more recent literature is 79–97 %, compared with only 52–56 % by mammography. The sensitivity reaches 98 % in high-grade or comedo type DCIS [16, 17]. Several recent studies investigated the utility of MRI in the detection of invasive component in DCIS diagnosed on needle biopsies. The presence of a mass, rapid initial enhancement, washout kinetics, larger lesion size, higher lesion to background signal intensity ratios, higher number of tissue cores involved by tumor nests, and lower apparent diffusion coefficient (ADC) values have been linked to the presence of occult invasion [18–20].
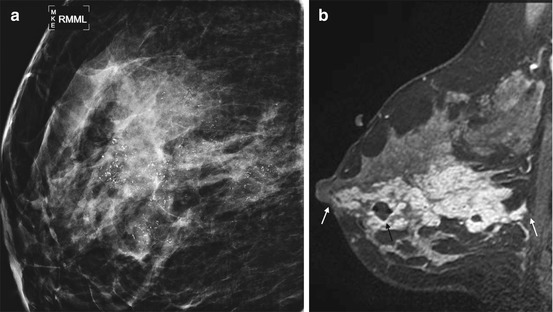

Fig. 4.4
Clinical stage 0, TisN0M0 tumor in a 39-year-old woman with extensive DCIS. (a) Spot magnification mediolateral view mammogram of the right breast demonstrates dense breast tissue with extensive pleomorphic microcalcifications that did not extend to the nipple. Biopsy confirmed high grade DCIS. (b) Sagittal post contrast fat-saturated T1 MR image shows extensive clumped nonmass enhancement. The tumor extends to within 2 mm of the nipple anteriorly and 3 mm of the pectoral muscle posteriorly (white arrows). A small hematoma from biopsy is present (black arrow). The patient is not an appropriate candidate for nipple-sparing mastectomy because the close proximity of tumor to the nipple suggests occult nipple invasion
4.3 Additional Sites of Disease
Multifocal disease is defined as two or more tumor foci in the same quadrant of the breast (Fig. 4.5). Multicentric disease is a condition with two or more tumor foci in different quadrants of the breast (Fig. 4.6). Although TNM staging system does not take these into consideration, the detection of additional sites of disease greatly impacts surgical management. While multifocal disease may be amenable to breast conservation, multicentric disease is usually treated with mastectomy. MRI is superior to conventional imaging for identifying additional cancer foci in the same breast as the index tumor, and in the opposite breast [21–26]. The preoperative identification of these additional tumor foci may alter surgical and radiation therapy. In a recent meta-analysis of 50 studies, Plana and associates found that preoperative MRI detected additional, otherwise occult, cancers in the ipsilateral breast in 20 % of cases, with a summary positive predictive value (PPV) of 67 % and accuracy of 93 %. The PPV increased to 75 % when MR scanner ≥1.5 T was used [23]. These results are similar to the findings of an earlier meta-analysis of 19 studies, showing detection of additional disease in 16 % of cases with a summary PPV of 66 % and accuracy of 86 % [24]. In this and another meta-analysis, MRI found additional cancer in the contralateral breast in 4.1–5.5 % of patients (Fig. 4.7) at the time of diagnosis [23, 25]. This is similar to the 3.1 % rate reported by the ACRIN 6667 multicenter prospective trial [26].
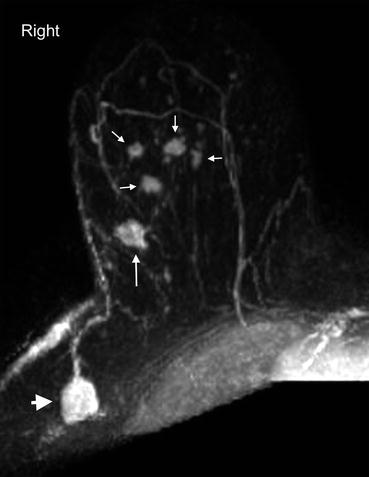
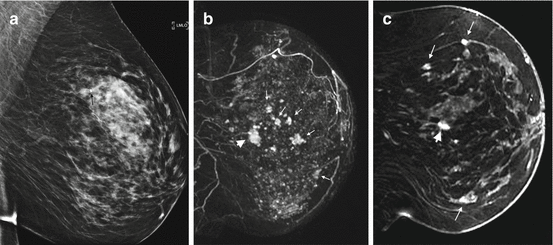
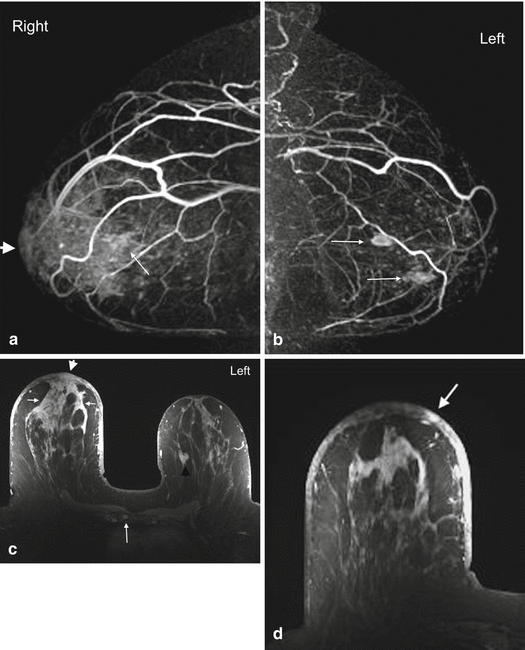

Fig. 4.5
Clinical stage IIA, T1N1M0 tumor in a 52-year-old woman with multifocal carcinoma. Axial post contrast subtraction T1 MIP MR image of the right breast demonstrates multiple enhancing masses in the central and lateral aspects of right upper breast. The largest, 1.2 cm mass is a known invasive carcinoma (long arrow). Four additional tumors (short arrows) are seen anterior to it. An enlarged level I right axillary lymph node with loss of reniform shape and fatty hilum (arrowhead) was positive for metastatic disease on fine needle aspiration

Fig. 4.6
Clinical stage IIA, T1N0M0 tumor in a 48-year-old woman with multicentric tumors. An architectural distortion on her screening mammogram led to the ultrasound biopsy of a 1.2 cm mass, which revealed invasive lobular carcinoma. (a) Left MLO view mammogram shows heterogeneously dense breast with a biopsy marker at the site of the index tumor (black arrow). No other suspicious abnormality is visible. (b) Sagittal post contrast T1 MIP MR image demonstrates the lobulated index mass (arrowhead) and multiple additional small irregular enhancing masses (small arrows). (c) Sagittal post contrast fat-saturated T1 MR image shows part of the known index tumor (arrowhead). Multiple tumors in different quadrants (long arrows) are better appreciated. Biopsies of two additional masses confirmed multicentric invasive lobular cancers

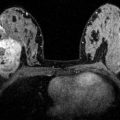
Fig. 4.7
Clinical stage IV, T4dN1M1 tumor in a 63-year-old woman with diffuse erythema of the right breast. Skin punch biopsy confirmed inflammatory breast cancer (IBC). Staging MRI showed contralateral left breast cancers and positive right axillary level I, II nodes. (a) Sagittal T1 MIP MR image of the right breast reveals extensive nonmass enhancement (between arrow and arrowhead) and enhancement of the nipple consistent with invasion (arrowhead). A partially obscured irregular mass is seen more posteriorly (long arrow). (b) Sagittal T1 MIP MR image of the left breast shows two masses with heterogeneous enhancement. Biopsy confirmed both to be invasive ductal carcinoma. (c) Bilateral axial post contrast fat-saturated T1 MR image demonstrates asymmetric enlargement of the right breast with diffuse thickening and heterogeneous enhancement of the skin. Nipple invasion (white arrowhead) and extensive subareolar nonmass enhancement (between short arrows) are obvious. An enhancing mass is seen in the left breast (black arrowhead). An enhancing focus in the right sternum (long white arrow) was positive on PET/CT scan, consistent with distant metastasis. (d) Axial post contrast fat-saturated T1 MR image of the right breast demonstrates enhancing nodule in the skin (arrow) caused by dermal lymphatic embolus (the “punched out” lesion). Diffuse thickening and heterogeneous enhancement of the skin are evident
Many studies have examined the surgical impact of finding additional sites of disease. Plana’s meta-analysis of 26 studies found an appropriate change in surgical management in 12.8 % of patients with confirmed additional malignancy, with 8.3 % of patients converted from breast conservation therapy (BCT) to mastectomy and 4.5 % receiving more extensive excision [23]. However, false positive cases resulted in inappropriate alteration in surgical treatment in 6.3 % of cases, including 1.7 % undergoing mastectomy and 4.6 % receiving more extensive excision [23]. These results parallel the findings of another meta-analysis, which showed a 1.1 % conversion rate to mastectomy and a 5.5 % rate of more extensive surgery due to false positive MRI [24]. The false positive cases illustrate the importance of histologic confirmation of suspicious MRI findings before performing more extensive surgery.
Occasionally, additional tumor may be present, but not detected by MRI. These false negative cases may be caused by non-enhancing tumor or obscuration by moderate to marked background parenchymal enhancement (BPE) of normal tissue [2, 27, 28]. BPE is mediated by hormonal activity, and is not correlated with mammographic density [28]. Attempts should be made to schedule breast MRI during the second week of the menstrual cycle or discontinuing exogenous hormone therapy for several months before MRI to reduce BPE. However, to avoid delay in therapy, this is not possible in patients newly diagnosed with breast cancer.
4.4 Pectoral Muscle and Chest Wall Involvement
Knowledge of pectoral or chest wall invasion by breast cancer prior to surgery is important, because of its impact on tumor staging, surgical planning and overall therapeutic approach. Chest wall invasion is defined as tumor infiltration of ribs, intercostal muscles and/or serratus anterior muscle [29]. Breast tumor with chest wall invasion is considered locally advanced disease with a tumor classification of T4a and a minimum TNM stage of IIIB with a 5-year survival rate of 23 % [30, 31]. Breast tumor with chest wall invasion may require neoadjuvant chemotherapy, with or without chest wall radiation, followed by more extensive surgery including chest wall resection [30, 31]. A tumor that invades only the pectoral muscle may require partial excision of the muscle if the invasion is superficial, or radical mastectomy with resection of the entire muscle if full thickness of the muscle is involved (Fig. 4.8) [30].
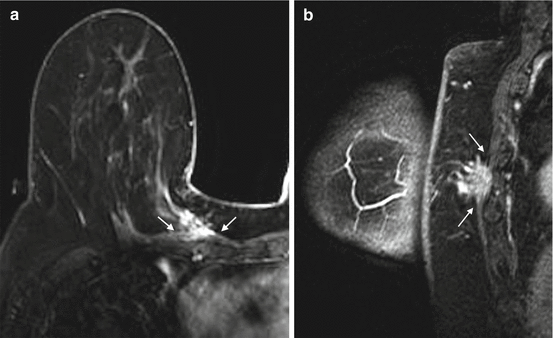

Fig. 4.8
Clinical stage IIA, T2N0M0 tumor in a 70-year-old woman with invasive ductal carcinoma of the right breast. (a) Axial post contrast fat-saturated T1 MR image demonstrates a posteriorly located tumor in the right breast with full thickness involvement of the pectoral muscle (between arrows). The tumor has a maximum dimension of 2.2 cm. (b) Sagittal post contrast fat-saturated T1 MR image shows the irregular mass invading the pectoral muscle (between arrows) without affecting the underlying intercostal muscles
Evaluation of the pectoral muscle and chest wall underlying a posteriorly located breast tumor is usually limited on physical examination, mammography and ultrasound [30–32]. Far posterior tumors are difficult to include in the field of view on mammography. On sonography, the strong acoustic shadowing by breast cancer often obscures the underlying pectoral muscle. By contrast, the pectoral muscle and chest wall are well demonstrated on MRI (Fig. 4.9) [32]. Previous studies showed that contrast enhancement of the pectoral muscle or chest wall structures, either infiltrative or mass-like (Figs. 4.8 and 4.10a), are the only reliable MRI finding to predict invasion [32, 33]. Proximity of the tumor or violations of the fat plane alone are not sufficient evidence of muscle invasion (Fig. 4.11a) [32, 33]. Pectoral muscle enhancement caused by recent biopsy of nearby primary tumor is a known cause of false positive interpretation [33].
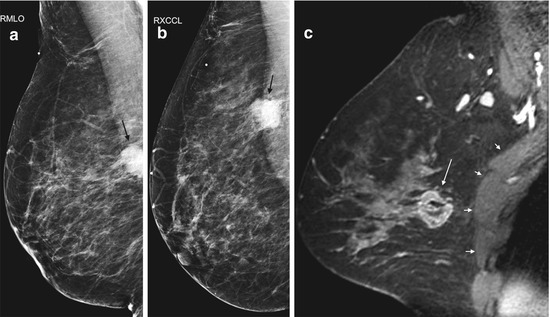
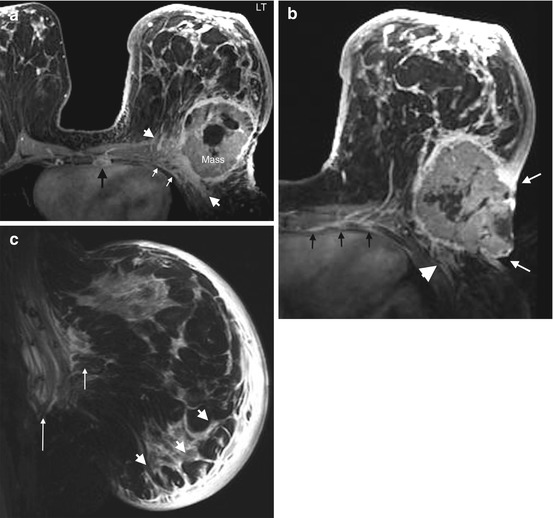
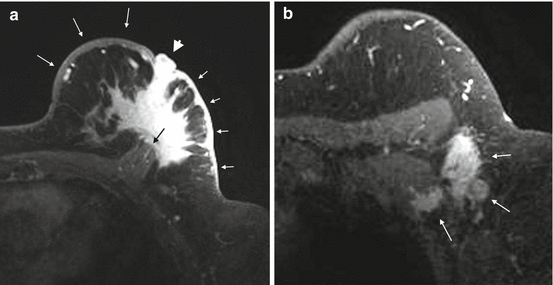

Fig. 4.9
Clinical stage IIA, T2N0M0 tumor in a 44-year-old woman with a posteriorly located invasive ductal carcinoma of the right breast. (a) Right MLO view mammogram demonstrates a 2.5 cm mass (black arrow) in the posterior breast, incompletely imaged and inseparable from pectoral muscle. A BB on the breast skin denotes a palpable mass. (b) On the laterally exaggerated CC view, the tumor again overlaps with the pectoral muscle. (c) Sagittal post contrast fat-saturated T1 MR image shows the mass (long arrow) not in close proximity or invading the pectoral muscle (four small arrows)

Fig. 4.10
Clinical stage IIIC, T4dN3Mx tumor in a 41-year-old woman with triple-negative invasive ductal carcinoma, and clinical evidence of inflammatory carcinoma of the left breast. (a) Axial post contrast fat-saturated T1 MR image demonstrates a 10 cm left breast mass with enhancement of the pectoral muscle, indicating invasion (between arrowheads). Enhancement of intercostal muscles and pleura (small white arrows) indicates chest wall invasion. An enlarged left internal mammary lymph node (black arrow) and palpable left axillary nodes constitute N3 nodal status. Diffuse thickening and enhancements of the skin and Cooper ligaments are consistent with inflammatory carcinoma. (b) Axial post contrast fat-saturated T1 MR image shows the locally advanced tumor invading the skin with ulceration (between small white arrows), the pectoral muscle (arrowhead) and the intercostal muscle (black arrows). (c) Sagittal fat-saturated T2 image demonstrates diffuse cutaneous and subcutaneous edema (arrowheads), prepectoral edema (short arrow), and intramuscular edema (long arrow). These are differential features in favor of IBC

Fig. 4.11
Clinical stage IIIA, T3N2M0 tumor in a 54-year-old woman with a left breast mass and left nipple retraction. (a) Axial post contrast fat-saturated T1 MR image of the left breast reveals a large (7.1 cm) spiculated enhancing mass abutting the pectoral muscle (black arrow). Obliteration of the muscle fascia and tenting of the muscle are present, but there is no muscle enhancement to indicate invasion. Enhancement and retraction of the nipple (arrowhead) indicates nipple invasion. Diffuse thickening and enhancement of the skin in the lateral aspect of the breast (four short arrows) signal skin invasion by local extension. Skin thickening without enhancement in the medial breast (three long arrows) reflects lymph edema without invasion. (b) Axial post contrast fat-saturated T1 MR image more superiorly reveals three abnormal level I axillary nodes lying lateral to the pectoralis muscles. The two lateral nodes are matted to each other, while the medial node adheres to the pectoralis minor muscle. Note the loss of hilar fat in the nodes. Spiculated margins of the nodes suggest extracapsular tumor extension, which was confirmed by core biopsy
4.5 Skin and Nipple Involvement
According to AJCC TNM system for clinical staging of breast cancer, ulceration and/or satellite nodules and/or edema (including peau d’orange) of the skin which do not meet the criteria for inflammatory carcinoma, are classified as T4b tumor, resulting in at least stage IIIB disease (Tables 4.1 and 4.4). Invasion of the dermis alone, without the above mentioned skin changes, does not meet the criteria of a T4 tumor (Table 4.1). On MRI, direct invasion of the skin appears as localized skin thickening and enhancement, which is contiguous with an underlying malignancy, with or without skin retraction (Fig. 4.11a). Skin edema, seen as areas of non-enhancing skin thickening (>3 mm) on MRI, may occur as a result of lymphatic obstruction, with or without malignant involvement (Fig. 4.11a). In later stages, enhancing skin nodules, masses, and ulceration are well demonstrated on MRI (Fig. 4.10b). When skin involvement by a locally advanced tumor is extensive, differentiating it from inflammatory carcinoma on clinical examination and MRI is difficult without a skin punch biopsy [34].
Preoperative evaluation of the nipple-areolar complex (NAC) is important for surgical planning because involvement of the NAC by tumor requires resection of the NAC and precludes patient from nipple-sparing mastectomy. Assessment of the NAC for tumor involvement on MRI may be difficult, because normal nipples may show various patterns of enhancement or no enhancement at all [28]. Sakamoto and colleagues found unilateral nipple enhancement continuous with the underlying index tumor to be highly suggestive of tumor involvement (Figs. 4.2, 4.3, 4.7 and 4.11) [35]. Characteristics of the nipple enhancement include diffuse enhancement, periareolar skin enhancement, and rim or periductal enhancement within the nipple [35]. Nodular enhancement in the involved nipple is occasionally seen (Fig. 4.3b). Tumor size >2 cm and distance from the tumor edge to the NAC < 2 cm on MRI are statistically significant indicators for NAC involvement [36]. However, the tumor to NAC distance indicative of nipple involvement has been reported as <5 mm or <10 mm in other studies (Fig. 4.4) [37, 38]. Moon and associates found enhancement of the NAC itself to have higher predictive value for NAC invasion than short tumor to nipple distance [39].
4.6 Staging of Regional Lymph Nodes
Identification of regional nodal metastases is critical for staging, prognosis and treatment planning in patients with newly diagnosed breast cancer (Table 4.2). Regional lymph nodes include ipsilateral intramammary, axillary, internal mammary, and supraclavicular nodes.
The axilla is divided into 3 levels by the pectoralis minor muscle. Level I nodes are low axillary nodes lateral to pectoralis minor muscle, including the intramammary nodes (Fig. 4.12a). Level II nodes are mid-axillary nodes between the medial and lateral borders of the pectoralis minor muscle, including the Rotter nodes between the pectoralis major and minor muscles (Figs. 4.12b and 4.13a). Level III nodes are apical axillary nodes medial to the pectoralis minor muscle, i.e. the infraclavicular nodes (Fig. 4.12c). The internal mammary nodal chain runs along the margins of the sternum following the course of internal mammary artery and vein (Fig. 4.13b and 4.13c). The internal mammary (IM) nodes are found in the first through sixth intercostal spaces [40]. The supraclavicular nodes are located in the supraclavicular fossa.
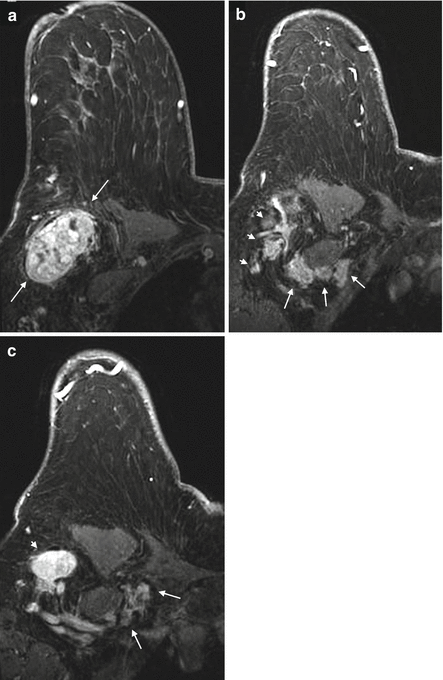
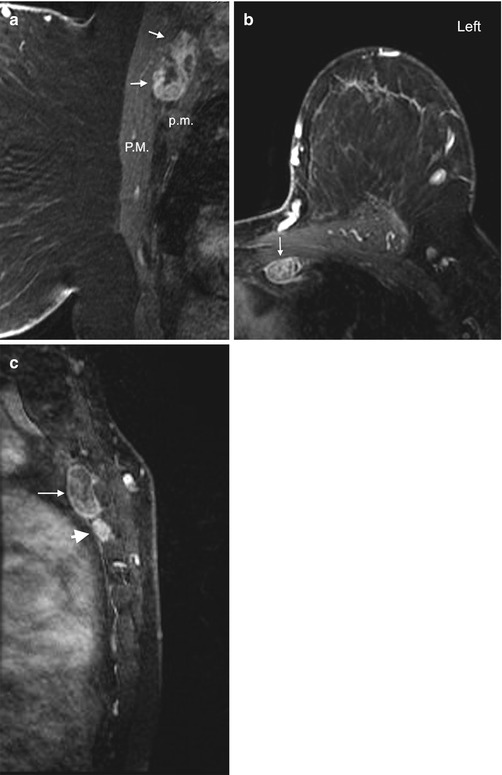

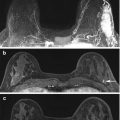
Fig. 4.12
Clinical stage IIIC, TxN3M0 tumor in a 75-year-old woman with right axillary lymphadenopathy and no apparent primary tumor on mammography and ultrasound. (a) Axial post contrast T1 fat-saturated MR image shows a large right axillary level I lymph node with heterogeneous enhancement, complete absence of hilar fat and perinodal stranding which may be due to recent biopsy or lymph edema. Biopsy of this node revealed poorly differentiated mammary carcinoma. (b) Axial image at a higher level reveals multiple level II nodes posterior to the pectoralis minor muscle (long arrows). Some level I nodes lying lateral to the pectoralis minor muscle are seen (arrowheads). All nodes show ill-defined margins suspicious for extranodal tumor extension. (c) Axial image at the level of infraclavicular fossa demonstrates matted level III lymph nodes medial to the pectoralis minor muscle (between arrows). An abnormal level I node is seen (arrowhead). No primary tumor is identified in either breast

Fig. 4.13
Interpectoral node and internal mammary (IM) nodes. (a) Sagittal post contrast fat-saturated T1 image shows an enlarged lymph node (arrows) with heterogeneous enhancement between the pectoralis major (P.M.) and pectoralis minor (p.m.) muscles. The interpectoral node is also known as a Rotter node. (b) Axial post contrast fat-saturated T1 image of left breast in a different patient demonstrates an enlarged left IM node (arrow). (c) Sagittal post contrast T1 image of the same patient as in image (b) shows the IM node (arrow) along the sternal border. A second abnormal IM node is seen inferior to it (arrowhead)
The current 7th edition of the AJCC TNM staging system includes clinical and pathologic node staging schemes [1, 41]. The “clinical” scheme classifies “clinically detected” nodes, which are defined as nodes detected by clinical examination and imaging studies. The “pathologic” scheme classifies nodes identified with sentinel node biopsy or axillary node dissection. In the clinical scheme (Table 4.2), ipsilateral level I and II axillary nodes are N1 disease if movable, but become N2 disease when fixed to each other or adjacent structures (i.e. matted), which raises the stage to at least IIIA (Table 4.4). Metastases in the ipsilateral IM nodes in the absence of axillary node metastases are classified as N2 disease, but become N3 disease if the axillary nodes are also involved. Metastasis to the ipsilateral level III axillary (infraclavicular) or supraclavicular nodes indicates N3 disease, which raises the stage to at least IIIC. Metastases to cervical, contralateral internal mammary and contralateral axillary lymph nodes are considered distant metastases (M1 disease) and indicate stage IV disease (Table 4.4) [30]. Metastases to the IM nodes usually occur after a tumor has metastasized to the axilla (N3 disease). Isolated metastasis to the IM nodes is rare, occurring in only 1–5 % of breast cancers, usually from deep or medial lesions [41, 42]. Metastatic involvement of the IM nodes, without or with axillary disease, carries a small but definite risk of local recurrence and reduced long-term survival [42]. Due to the morbidity involved, dissection of the internal mammary nodes is usually not performed. However, radiation treatment can be utilized to treat these nodes [41, 42].
In most institutions, ultrasound is the primary imaging modality for evaluation of axillary nodes, with moderate sensitivity and high specificity for detection of metastases, especially when morphologic criteria rather than size, are used for diagnosis [41, 43, 44]. However, the results are operator dependent and the evaluation of infraclavicular, supraclavicular and internal mammary nodes is not routinely performed. By contrast, regional lymph nodes, except for supraclavicular nodes, are included in the field of view on most routine breast MRI protocols. The ability of MRI to predict axillary nodal metastases is similar to ultrasound, with reported sensitivity of 36–88 % and specificity of 73–100 % [45–50]. MRI is less operator-dependent than ultrasound and provides a global view of both axillae and internal mammary chains. This may enhance the detection of potentially abnormal nodes and allows comparison with the contralateral axilla [41]. Occasionally, pulsation artifacts through the axilla may limit evaluation of the axillary nodes [41].
On non-contrast MRI, normal lymph nodes are reniform, circumscribed, with low signal intensity on T1-weighted and high signal intensity on T2-weighted sequences. Hilar fat is best seen on a T1-weighted non-contrast sequence without fat saturation, a sequence that should be included in the breast MRI protocol. Upon contrast injection, the normal lymph nodes enhance rapidly and homogeneously with a type III wash out delayed kinetics. Hence, the enhancement kinetics are not useful in differentiating benign and metastatic lymph nodes. Like ultrasound, nodal size alone is not useful for identifying metastatic nodes on MRI [41]. Morphologic features on MRI that suggest a nodal metastasis include: round shape or a long axis to short axis ratio of less than two, loss of the fatty hilum, increased cortical thickness (>3 mm), eccentric or focal cortical thickening, irregular or spiculated margins, edema surrounding the nodes, and asymmetry of morphology of the nodes compared with the contralateral axilla [41, 50–53]. One study described “perifocal edema” (edema surrounding the lymph nodes) and “rim enhancement” (higher signal intensity in the periphery of the nodes) 11 min after contrast injection as the two features with 100 % positive predictive value for the detection of metastases [53]. IM nodes are more likely to contain a metastasis when 5 mm or larger in size [54]. Normal IM nodes are usually not visible on MRI. When visualized, they should be regarded as suspicious and reported [30].
Traditionally, preoperative identification of axillary nodal metastases will spare patients with invasive breast cancer an unnecessary sentinel lymph node biopsy (SLNB) and allow them to proceed directly to axillary lymph node dissection (ALND). In 2011, Giuliano and associates published the results of the American College of Surgeons Oncology Group (ACOSOG) Z0011 randomized trial [55]. This trial suggested that patients with T1 or T2 invasive breast cancer, no palpable nodes, and one or two positive sentinel nodes, who underwent lumpectomy with negative margins, tangential whole-breast radiation, and systemic therapy, might not benefit from ALND [55]. While this finding is potentially practice changing, controversies exist about the relatively short median follow-up interval of 6.3 years and the number of patients enrolled. In light of the Z0011 results, some have questioned the role of imaging for preoperative axillary staging, expressing concerns that preoperative detection of axillary metastasis would prompt ALND for disease that could otherwise have been treated according to Z0011 protocol [56]. Many authors believe that imaging still plays an important role in the axillary staging, especially in identifying patients with N2 and N3 disease. Since nodal disease beyond levels I and II are not routinely included in an axillary dissection, identification of nodes in these higher N categories by imaging may affect initial staging and treatment planning. Two recent studies showed that MRI can predict metastatic disease in more than two sentinel nodes, thereby identifying patients who require further local-regional therapy beyond SLND [57, 58]. In the future, patients may undergo imaging for the purpose of excluding N2 or N3 disease, rather than for diagnosing axillary metastases [41].
4.7 Subsets of Newly Diagnosed Breast Cancer Patients Likely to Benefit from MRI Staging
Because of the ability of MRI to identify lesions that are occult on conventional imaging and to better define extent of disease, it is intuitive that MRI staging is particularly beneficial for a subset of patients with newly diagnosed breast cancer.
Patients with Invasive Lobular Cancer (ILC)
ILC tends to present with multiple and bilateral tumor sites and is better detected with MRI than mammography. The reported sensitivity of MRI for detection of ILC, ranging from 93 % to 96 %, is significantly higher than the sensitivity of mammography, which is in the range of 34–81 % [2, 22, 59]. Further more, MRI is more accurate in assessing the extent of ILC than mammography, leading to lower re-excision rates for positive surgical margins [60, 61].
Patients at High-Risk for Developing Breast Cancer
Patients with Dense Breast Tissue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree