Fig. 3.1
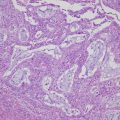
The major morphological types of epithelial ovarian tumors and the mimic normal epithelia; (a and b) fallopian tubal epithelium (a) and serous carcinoma (b); (c and d) endometrium in proliferative phase (c) and endometrioid carcinoma (d); (e and f) gestational endometrium with Arias-Stella reaction (e) and clear cell carcinoma (f); (g and h) endocervical epithelium (g) and mucinous carcinoma which recently is classified in seromucinous carcinoma (h); (i and j) intestinal epithelium (i) and mucinous carcinoma (j) [(a–j) hematoxylin and eosin staining; (a–j) ×200]
The source of EOTs has been a recent topic of debate [1, 2]. The past and current paradigm is that EOTs arise from the ovarian surface epithelium (OSE) covering the ovary and lining inclusion cysts which are derived from surface invaginations. OSE originating developmentally from the coelomic epithelium is composed of flat, nondescript cells morphologically similar to the mesothelium lining of the peritoneal cavity. The OSE is thought to be capable of metaplasia to a Müllerian phenotype resembling oviductal, endometrial, or endocervical epithelia, known as a secondary Müllerian system [3–5]. Thus, the OSE is suspected to carry pluripotent cells, i.e., putative stem cells. The recent identification of such stem cells implicates the OSE in the pathogenesis of EOTs [6, 7].
Other recent studies have indicated that a considerable number of EOTs originate in the fallopian tube and the endometrium, before migrating to the ovary. This theory, one of “imported disease”, is thought to be an influential paradigm shift in the morphological theory of EOTs. According to this theory, serous tumors arise from the implantation of epithelium from the oviduct, and endometrioid and clear cell tumors are associated with endometriosis that mainly develops from retrograde menstruation [4].
Clinical, morphological, and molecular studies have provided a model for malignant EOTs, with two broad categories designated as Type I and Type II. Type I carcinomas progress in an indolent course, are usually confined to the ovary at diagnosis, and are relatively genetically stable. Type I carcinomas exhibit a shared lineage with their corresponding benign and borderline-malignant tumors, supporting the concept of a morphological sequence of tumor progression. In contrast, Type II carcinomas are highly aggressive, progress rapidly, and are usually in an advanced stage at diagnosis. Type II carcinomas do not exhibit the shared lineage and are genetically unstable [8].
In the first section of this chapter, these important theories of ovarian tumorigenesis and the two-tiered system of classification are introduced and organized. Finally, the morphological and molecular details of four representative malignant EOTs, namely, serous carcinoma (high grade, low grade), endometrioid carcinoma, clear cell carcinoma, and mucinous carcinoma, will be presented and discussed.
3.2 Representative Theories Related to the Morphological Pathogenesis of EOTs
3.2.1 The Theory of the Secondary Müllerian System
In the first half of the twentieth century, it was believed that the OSE, which was also referred to as the germinal epithelium, carried pluripotent stem cells which differentiate to germ cells and follicular cells [9, 10]. Even now, it is thought that the OSE is derived from a common embryonic origin in the pluripotent coelomic epithelium which gives rise to the Müllerian ducts, i.e., the epithelia of the fallopian tubes, endometrium, uterine cervix, and upper part of the vagina. According to this theory, a subset of pluripotent OSE cells and cells lining the inclusion cysts have the propensity to differentiate along the lineage of the Müllerian epithelium [11], with this therefore being referred to as a secondary Müllerian system [12–14]. For example, serous metaplasia of OSE inclusion cysts is characterized by a cuboidal epithelium with cilia, which mimics the endosalpingeal epithelium [15] (Fig. 3.2a). With this in mind, the morphological alteration of the OSE and its inclusion cysts has been suggested as a potential site of origin for the development of EOTs [5] (see also Sect. 5.3 in Chap. 5).
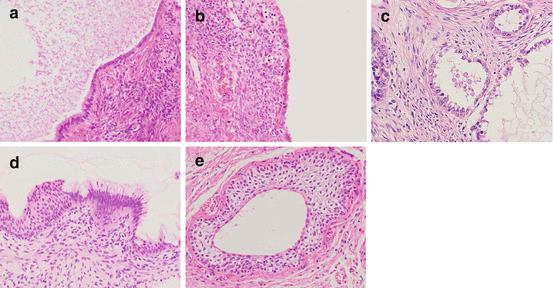

Fig. 3.2
Putative or possible sources of the major types of epithelial ovarian tumors. (a) inclusion cyst (cuboidal epithelium partially with cilia); (b) endometriosis; (c) adenofibroma (clear cell type); (d) teratoma (squamous cell and mucinous epithelia); (e) transitional cell (Walthard) nests [(a–e) hematoxylin and eosin staining; (a–e) ×200]
In regard to EOT tumorigenesis, the “incessant ovulation” hypothesis for ovarian cancer, which postulates that follicular rupture [16] and repair trauma increases OSE cell proliferation and risk of transformation, was proposed more than 40 years ago [17]. Besides primary endocrinological functions, gonadotropin hormones, such as follicle-stimulating hormone (FSH), luteinizing hormone (LH), and human chorionic gonadotropin (hCG), are thought to be involved in OSE cell proliferation and the repair of OSE following ovulatory trauma [18, 19]. Invagination and inclusion cysts form in the ovarian cortex as a result of the repair, and exposure of the entrapped cyst-lined OSE cells to foreign micro-substances within the cystic lumen, which come from the outside environment via the fallopian tube, causes their transformation [5]. In this process, stemlike cells undergo metaplasia and transformation to acquire a highly complex morphology resembling either the Müllerian duct-derived fallopian tube (serous type), the endometrium (endometrioid type), the endometrium with Arias-Stella reaction (clear cell type), or the endocervix (seromucinous or previously mucinous type) [20] (Fig. 3.1a–h).
Initially, the “incessant ovulation” hypothesis proposed for the stemlike cells of OSE was not widely accepted. The first scientific evidence for the existence of putative stem cells on the ovarian surface came in 2008, with a subset of stemlike cells experimentally identified by a stemness assay [6]. Subsequently, a subset of OSE cells expressing a common hematopoietic stem cell marker (Ly6a+) were identified in adult mouse ovaries [21], and more recent in vivo studies have used fate-mapping methodologies to provide direct evidence for the existence and location of self-renewing epithelial stem cells in the ovary [7, 22]. Stemlike OSE cells that display high aldehyde dehydrogenase (ALDH) activity [22] and high ALDH activity with expression of LGR5 (leucine-rich repeat-containing G protein-coupled receptor 5) [7] have been located in both the murine and human ovary hilum [20]. In view of such findings, it has been suggested that OSE stem cells might participate in postovulatory wound closure, as well as the tumorigenesis of EOTs [18, 23, 24].
3.2.2 The Theory of Imported Disease
Recent investigations have revealed that high-grade serous carcinomas are derived from the fimbriae of the fallopian tube. The theory of tubal involvement in the tumorigenesis of high-grade serous carcinoma proposes that serous tubal intraepithelial carcinomas (STICs) (Fig. 3.3a, b), which are known to occur in the fimbriae, are ectopically implanted into the ovarian stroma as cortical inclusion cysts, and that the exposure of these cells to the ovarian stromal microenvironment, which produces abundant growth factors designed for folliculogenesis, leads to “ovarian cancer” consisting of high-grade serous carcinoma [4, 8].
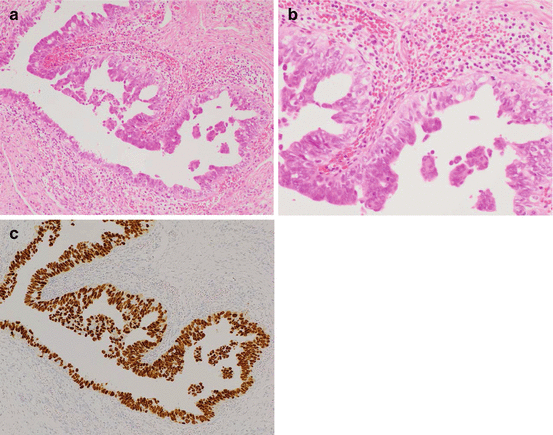

Fig. 3.3
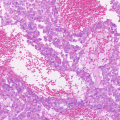
Serous tubal intraepithelial carcinoma [(a and b) hematoxylin and eosin staining; (c) p53 immunostaining; (a and c) ×100; (b) ×200]
In endometrioid and clear cell carcinoma, it has been well recognized that malignant transformation can occur at epithelial components of endometriosis in endometriotic cysts of the ovary. A follow-up program for endometriotic cysts confirmed the risk of malignant transformation resulting from endometriosis [25]. Endometriosis is thought to occur via retrograde menstruation, where endometrial epithelial cells and stromal cells move from the uterus through the fallopian tubes and subsequently become established as an endometriotic cyst [26, 27]. It is also known that retrograde stromal cells from the endometrium can implant to the ovary during menstruation, inducing a metaplastic change in the OSE and resulting in endometriosis [28]. This creates a unique microenvironment where menstruation-like blood are trapped within the cyst, resulting in high concentrations of iron in a confined space, subsequent oxidative stress, and a hypoxic environment that promotes DNA damage and the accumulation of mutations [29–32].
Such studies suggest that the fallopian tube epithelium (benign or malignant) can implant onto the ovary to give rise to both low-grade and high-grade serous carcinomas and that similarly, endometrial tissue can implant onto the ovary with resulting endometriosis, then undergoing malignant transformation into endometrioid and clear cell carcinoma. According to the theory of “imported disease”, these EOTs are not ovarian in origin therefore but rather represent “imported disease”, and it is logical to conclude that the only true primary ovarian neoplasms are germ cell and gonadal stromal tumors, analogous to the situation in the testis which does not have epithelial tumors [4] (see also Sect. 5.3 in Chap. 5).
3.3 Two-Tiered Classification for Clinical, Morphological, and Molecular Pathogenesis
EOTs can be classified into Types I and II, which correspond to two distinct models of clinical, morphological, and molecular pathogenesis [33]. Type I tumors develop slowly, in a stepwise manner, from premalignant conditions or borderline tumors, and include low-grade serous carcinomas, endometrioid carcinomas, clear cell carcinomas, and mucinous carcinomas. In contrast, Type II tumors grow rapidly and are typically found to have spread beyond the ovaries at presentation. The predominate Type II tumors are high-grade serous carcinomas, with the remainder being carcinosarcomas and undifferentiated carcinomas. It was originally thought, since these tumors are rarely associated with morphologically recognizable precursor lesions, that they develop de novo from ovarian inclusion cysts or the surface epithelium [34, 35]. More recently, however, it has been recognized that Type II tumors with pelvic dissemination include carcinomas arising from the epithelium of the fimbriae.
Molecular studies have revealed that distinct biological signatures, compatible with the Type I and Type II classification system, exist among EOT subtypes. Although Type I carcinomas lack mutations in the TP53 gene and have a stable genome, each morphological subtype exhibits a distinctive molecular profile. Moreover, Type I carcinomas typically exhibit a shared lineage with their corresponding benign and borderline-malignant tumors, supporting the concept of a morphological sequence of tumor progression. Type II carcinomas display TP53 mutations in 80% or more of cases and rarely harbor the mutations that are found in Type I carcinomas. Type II carcinomas are typically associated with chromosome aneuploidy or chromosomal copy number abnormality resulting from an inherent chromosomal instability [8].
3.4 Morphological and Molecular Pathogenesis in Four Representative Malignant EOTs
3.4.1 Serous Carcinoma
3.4.1.1 High-Grade Serous Carcinoma
High-grade serous carcinoma is the most common type of malignant EOT and is classified as Type II. Morphologically, the tumor cells of high-grade serous carcinoma resemble the secretory cells of three distinct cell types from the fallopian tube epithelium, namely, secretary cells, ciliated cells, and peg cells [36–38]. Almost all of these tumors express the transcription factor PAX8 that is a marker of the secretory cell lineage in the fallopian tube epithelium. Until recently, all high-grade serous carcinomas were presumed to arise de novo in ovarian inclusion cysts or the OSE, although identification of putative precursors in these tissues had previously been difficult. Since the discovery of the tumor suppressor genes BRCA1 [39] and BRCA2 [40] (BRCA1/2) in 1994 and 1995, respectively, the use of mutation analysis in healthy women with a family history of hereditary breast and ovarian cancer (HBOC) syndrome has increased rates of prophylactic bilateral salpingo-oophorectomy. These surgical specimens have revealed that a subset of the fimbrial epithelium of the fallopian tube have lesions of occult carcinoma and STIC without any lesions in the ovary [41, 42]. It has also been reported that STIC of the fimbriae is concomitant with high-grade serous carcinoma of the ovary in sporadic but not only germline types and that STIC lesions have the same TP53 mutations as the ovarian lesions. The TP53 mutation findings indicate that there is clonal expansion between STIC and high-grade serous carcinoma of the ovary [43]. In fact, p53 immunopositivity by TP53 mutation in STIC is occasionally found in cases with high-grade serous carcinoma (Fig. 3.3). Furthermore, it has been revealed that small foci of p53-immunoreactive cells exist in largely histologically normal fallopian tube epithelium [36]. These foci, which predominate in the distal portion of the fallopian tube, have been designated “p53 signatures”. These p53 signatures probably represent early clonal expansion [44] and are found at the same frequency in women with or without BRCA1/2 mutation [36]. TP53 mutation is thus one of the earliest events in the genesis of high-grade serous carcinoma and may occur first in the discrete foci that lead to STIC in the distal fallopian tube. Extensive investigations have now examined the role of the fallopian tube in pathogenesis of the serous type of EOTs [36, 43, 45–47], yet it is clear that at least a subset of ovarian high-grade serous carcinomas do not have STIC involvement. Therefore, it is still considered that OSE may be a candidate as the site of origin for high-grade serous carcinoma without STIC. The exact proportion of tumors of ovarian and tubal derivation in cases of high-grade serous carcinoma could be revealed with the widespread implementation of an established pathology protocol for sectioning and examination of the fimbriae [46].
In high-grade serous carcinomas of the ovary including sporadic and hereditary types, TP53 gene mutations are found in 95% or more of cases [48, 49]. Mutations in several other tumor suppressor genes and oncogenes, such as NF1, RB1, and CDK12, have been reported, but their mutation frequency is low [49–51]. As somatic mutations in BRCA1/2 are known to be uncommon in sporadic serous tumors, it is possible that these genes are inactivated by mechanisms (loss of heterozygosity and/or methylation) other than mutation [52–54]. The proposed model is that loss of p53 and BRCA1/2 are early events that lead to a deficiency in homologous recombination repair of DNA double-strand breaks, triggering chromosomal instability and widespread copy number changes [34, 44, 49, 55–61]. The most common amplifications affect the genes MYC, CCNE, and MECOM, each of which is highly amplified in more than 20% of high-grade serous carcinomas [49], but it is the MYC gene that is the most often amplified and overexpressed [62]. In regard to other cancer-associated pathways harboring mutations, copy number changes, or changes in gene expression, the RB and phosphoinositide 3-kinase (PI3K)/RAS pathways are deregulated in 67% and 45% of high-grade serous carcinomas, respectively [49]. Various experimental models using OSE cells or tubal epithelial cells have supported the concept that molecular pathways based on TP53 mutation play an important role for the carcinogenesis of high-grade serous carcinomas or Type II tumors [23, 63, 64] (Fig. 3.4a) (see also Sect. 7.2.2 in Chap. 7).
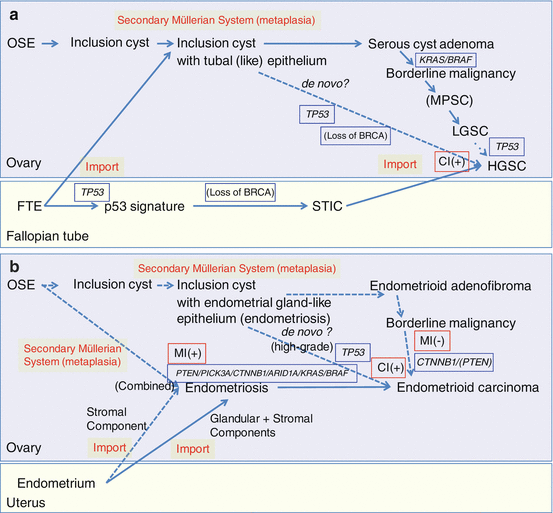
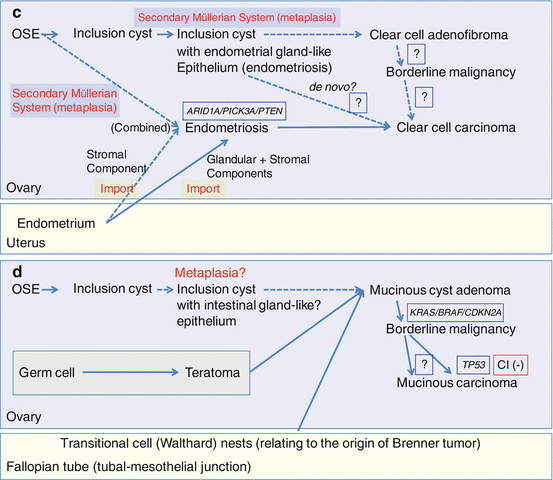


Fig. 3.4
Schematic diagram of morphological and molecular pathogenesis for ovarian carcinogenesis (a) serous tumors; (b) endometrioid tumors; (c) clear cell tumors; (d) mucinous tumors. Solid lines, major pathways; broken lines, minor or putative pathways; blue frames, gene mutations; red frames, genomic status. OSE, ovarian surface epithelium; FTE, fallopian tubal epithelium; STIC, serous tubal intraepithelial carcinomas; CI, chromosomal instability; MI, microsatellite instability; +, positive; −, negative
3.4.1.2 Low-Grade Serous Carcinoma
Low-grade serous carcinoma is much less common than high-grade carcinoma and is classified as a Type I tumor. These carcinomas frequently have a component of serous borderline tumor (SBT) or micropapillary serous carcinoma [65] and are thought to evolve in a stepwise fashion from benign serous cystadenoma through to SBT and finally to carcinoma. Morphologically, low-grade serous carcinomas also resemble tubal secretary cells and show small papillae of tumor cells exhibiting uniform nuclei within variable amounts of hyalinized stroma, which often contains psammoma bodies [66]. Low-grade serous carcinomas, like high-grade serous carcinomas, typically express the transcription factors PAX8 [67–69].
Low-grade serous carcinomas arise via the transformation of benign and SBTs, thought to be derived either from inclusion cysts originating from the OSE or from tubal epithelium that is shed and implanted onto the ovary and gives rise to inclusion cysts and subsequent serous neoplasms (Fig. 3.2a). An immunohistochemical study has shown that 80% of ovarian cortical inclusions express PAX8, a Müllerian marker, but not calretinin, a mesothelial marker, findings that support the concept of a tubal phenotype [70]. Recently, it has also been suggested that papillary tubal hyperplasia may be a putative precursor lesion for SBTs [71, 72].
Low-grade serous carcinomas are not associated with BRCA1/2 germline mutation and rarely have TP53 mutations, in contrast to KRAS and BRAF mutations which are frequently present. KRAS and BRAF are the upstream regulators in the RAS/RAF/MEK/ERK/MAP signal transduction pathway, which plays a critical role in the transmission of growth signals to the nucleus [73]. Oncogenic mutations in BRAF and KRAS result in the constitutive activation of this pathway and thus contribute to neoplastic transformation. Several studies have demonstrated that activating mutations in codon 12 (and less commonly in codon 13) of KRAS or in codons 599 and 600 of BRAF occur in approximately two thirds of SBTs and low-grade serous carcinomas [74, 75]. Mutations in KRAS and BRAF are mutually exclusive, such that tumors with mutant KRAS do not have mutant BRAF and vice versa. It has been suggested that mutations of KRAS and BRAF are early events associated with the initiation of SBTs and low-grade serous carcinomas and that a small subset of serous cystadenomas that acquire KRAS or BRAF mutations may progress to SBTs. Low-grade serous carcinomas do not show chromosomal instability and thus lack the complex genomic abnormalities seen in high-grade serous carcinomas (Fig. 3.4a) (see also Sect. 7.6 in Chap. 7).
3.4.2 Endometrioid Carcinoma
In 1927, Sampson was the first to describe the malignant transformation of endometriosis to ovarian carcinoma [76]. Thereafter, many studies have provided supporting evidence that malignant transformation can occur in ovarian endometriosis or the endometriotic cyst [77, 78] (Fig. 3.2b). The observation of a morphological transition from endometriosis to carcinoma in over one third of endometrioid carcinomas has led to endometriosis being considered its likely cause. It has thus been accepted that atypical endometriosis at the transition site is the precursor lesion for endometrioid carcinoma associated with endometriosis, and common genetic alterations have been documented in adjacent endometriosis, atypical endometriosis, and carcinoma [79]. Besides endometriosis, the coexistence of benign endometrioid neoplasms, such as adenofibromas or borderline tumors, with endometrioid carcinomas has been also recognized [80].
Endometriosis is thought to occur via retrograde menstruation, whereby epithelial and stromal cells of the endometrium are carried from the uterus through the fallopian tubes and can establish as an endometriotic cyst within the ovary [81]. Recent investigations suggest that the endometriotic cyst, in which chocolate-like blood is trapped for long time, maintains high concentrations of iron in the cystic fluid and that the iron-rich environment causes oxidative stress and hypoxia leading to DNA damage and accumulation of mutations [30, 32].
Like endometrial cancers, ovarian endometrioid carcinoma is commonly encountered in patients with Lynch syndrome. Microsatellite instability has been also observed in 13–20% of endometrioid carcinomas. Mutations in the PTEN tumor suppressor gene, resulting in the activation of PI3K signaling and inhibition of apoptosis, have been reported in a fifth or less of endometrioid carcinomas and are rare in other types of malignant EOT [82, 83]. Mutations in PIK3CA, which encodes the p110 catalytic subunit of PI3K, have also been identified in a fifth of endometrioid carcinomas and similarly result in activation of PI3K signaling [84, 85]. PTEN and PIK3CA mutations co-occur in a subset of endometrioid carcinomas [86, 87]. The Wnt/β-catenin signaling pathway is involved in the regulation of several important cellular processes, including cell fate determination, proliferation, motility, and survival. Mutations in CTNNB1, which encodes β-catenin, are typically found in endometrioid carcinomas but are uncommon in the other types of ovarian carcinoma [88], and several studies have noted the association of CTNNB1 mutation with squamous differentiation.
The tumor suppressor gene ARID1A, which encodes BAF250a, plays a crucial role in chromatin remodeling as a member of the SWI/SNF chromatin remodeling complex. ARID1A mutation induces changes in the expression of multiple genes (CDKN1A, SMAD3, MLH1, and PIK3IP1) as the result of chromatin remodeling dysfunction and has been shown to contribute to molecular pathogenesis and cellular transformation in cooperation with the PI3K pathway [82, 83, 86, 89–92]. KRAS and BRAF mutations have been identified in endometrioid carcinomas, but the frequency of these mutations is rather low, being 7% or less [92–96]. The fact that PTEN, KRAS, and ARID1A mutations are also found in the epithelial components of endometriosis adjacent to endometrioid carcinomas provides additional evidence for the precursor role of endometriosis in the molecular pathogenesis of ovarian endometrioid carcinomas [29, 97, 98]. In regard to TP53, mutations have been reported in poorly differentiated or high-grade endometrioid carcinomas. TP53 mutations are uncommon in tumors with Wnt/β-catenin and/or PI3K/PTEN signaling defects [96].
Using genetically engineered mice, experimental models of endometrioid tumor have now been developed. In one approach, simultaneous activation of KRAS and inactivation of PTEN in the OSE resulted in the development of ovarian tumors resembling human endometrioid carcinomas associated with endometriosis [99]. In another approach, conditional bi-allelic inactivation of APC and PTEN in the OSE promoted ovarian endometrioid tumors harboring Wnt and PI3K pathway defects comparable to human endometrioid carcinomas [92]. Furthermore, conditional inactivation of one or both ARID1A alleles in the OSE concurrently with APC and PTEN inactivation in these mice induced endometrioid tumors with morphological features similar to those of human endometrioid carcinoma [100] (Fig. 3.4b) (see also Sect. 7.4.1 in Chap. 7).
3.4.3 Clear Cell Carcinoma
The morphological characteristics of clear cell carcinoma are multiple complex papillae, densely hyaline basement membrane material expanding the cores of these papillae, and hyaline bodies. Mitotic figures are less frequent than in other types of ovarian carcinoma. As is the case for endometrioid carcinoma, there is a close association between endometriosis and clear cell carcinoma [101, 102]. The coexistence of adenofibromas or borderline tumors, with clear cell carcinomas, has been also recognized, being distinct from those arising from endometriosis [103, 104] (Fig. 3.2c).
Hepatocyte nuclear factor-1β (HNF-1β) is upregulated in clear cell tumors, including benign tumors, borderline tumors, and carcinomas [105], and thus most clear cell carcinomas are positive for HNF1-β [106, 107]. This transcription factor is expressed in the mid-to-late secretory and gestational endometrium with Arias-Stella reaction, atypical and inflammatory endometriosis, and clear cell carcinoma [105]. HNF-1β regulates several genes such as dipeptidyl peptidase IV (involved in the control of glycogen synthesis [108]), glutathione peroxidase 3, and annexin A4 [109]. The fact that HNF-1β is important in controlling multiple genes involved in glucose and glycogen metabolism suggests that upregulation of this factor may be responsible for the characteristic morphological feature of clear cell carcinoma, namely, a glycogen-rich cytoplasm with clear appearance [108, 110].
Mutations involving PI3K/PTEN signaling are common in clear cell carcinomas, with PIK3CA mutations reported in 20–25% of tumors and PTEN mutations in 8% of tumors [84, 86, 98]. Recently, it has been found that nearly half of clear cell carcinomas carry ARID1A mutations and lack BAF250 protein [97]. The occurrence of somatic mutations of PTEN and ARID1A in a subset of ovarian endometriotic cysts, within both tumor tissue and adjacent endometriosis, but not in distant endometriosis sites, suggests shared molecular alterations between clear cell and endometrioid carcinomas of the ovary and their putative precursor lesion [98]. This finding also suggests that PTEN and ARID1A inactivation occurs early during the malignant transformation of endometriosis [97].
Clear cell carcinomas do not appear to share other genetic changes with endometrioid carcinomas. Wnt signaling pathway defects and microsatellite instability, for example, have not been observed with significant frequency in these tumors [86, 111, 112] (Fig. 3.4c) (see also Sects. 7.3.3 and 7.3.4 in Chap. 7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree