Monoclonal gammopathy of undetermined significance (MGUS) is characterized by an M spike less than 3 g/dL and a bone marrow containing fewer than 10% plasma cells without evidence of CRAB (hypercalcemia, renal insufficiency, anemia, or bone lesions). Light chain MGUS has an abnormal free light chain (FLC) ratio, increased level of the involved FLC, no monoclonal heavy chain, and fewer than 10% monoclonal plasma cells in the bone marrow. Smoldering multiple myeloma has an M protein of at least 3 g/dL and/or at least 10% monoclonal plasma cells in the bone marrow without CRAB features.
Key points
- •
Monoclonal gammopathy of undetermined significance (MGUS) is characterized by an M spike less than 3 g/dL and a bone marrow containing fewer than 10% plasma cells and no evidence of CRAB (hypercalcemia, renal insufficiency, anemia, or bone lesions). It progresses at a rate of 1% per year.
- •
Light chain MGUS (LC-MGUS) is characterized by an abnormal free light chain (FLC) ratio, increased level of the involved FLC, no monoclonal heavy chain, and fewer than 10% monoclonal plasma cells in the bone marrow.
- •
Smoldering multiple myeloma (SMM) has an M protein of at least 3 g/dL and/or at least 10% monoclonal plasma cells in the bone marrow but no evidence of CRAB features. The risk of progression is 10% per year for the first 5 years and then decreases to 3% per year for the next 5 years and finally to 1% to 2% per year for the following 10 years.
Introduction
More than a half century ago, Jan Waldenström described patients with a small serum protein electrophoretic spike without evidence of multiple myeloma (MM), Waldenström macroglobulinemia (WM), or related disorders as having an essential hyperglobulinemia. He noted the constancy of the size of the protein spike and utilized the term benign monoclonal gammopathy. In contrast, he emphasized that patients with MM or WM had an increasing quantity of monoclonal protein that produced symptomatic disease.
The term monoclonal gammopathy of undetermined significance (MGUS) was introduced more than 35 years ago, because patients with apparently benign monoclonal gammopathy were at risk for the development of symptomatic MM, WM, Light-chain (AL) amyloidosis, or a related disorder during long-term follow-up. This article will focus on MGUS, and the related disorder, smoldering multiple myeloma (SMM).
Introduction
More than a half century ago, Jan Waldenström described patients with a small serum protein electrophoretic spike without evidence of multiple myeloma (MM), Waldenström macroglobulinemia (WM), or related disorders as having an essential hyperglobulinemia. He noted the constancy of the size of the protein spike and utilized the term benign monoclonal gammopathy. In contrast, he emphasized that patients with MM or WM had an increasing quantity of monoclonal protein that produced symptomatic disease.
The term monoclonal gammopathy of undetermined significance (MGUS) was introduced more than 35 years ago, because patients with apparently benign monoclonal gammopathy were at risk for the development of symptomatic MM, WM, Light-chain (AL) amyloidosis, or a related disorder during long-term follow-up. This article will focus on MGUS, and the related disorder, smoldering multiple myeloma (SMM).
Recognition of monoclonal gammopathies
MGUS and SMM are asymptomatic and are incidentally discovered during work-up of a variety of clinical disorders and symptoms. Typically, a patient suspected to have a clonal plasma cell disorder is screened for the presence of a monoclonal (M) protein using serum protein electrophoresis, serum immunofixation, and the free light chain (FLC) assay. Serum protein electrophoresis is performed on agarose gel or capillary zone electrophoresis. The serum FLC is increasingly being used in place of urine studies during screening. However, if a serum M protein is found, electrophoresis and immunofixation of an aliquot from a 24-hour urine collection are also needed. The amount of M protein provides a measure of the patient’s tumor mass and is therefore useful in monitoring the course of one’s disease.
The presence of an M protein or an abnormal serum FLC ratio indicates the presence of MGUS, SMM, or a more serious process such as MM, WM, AL, or related plasma cell disorder. MGUS and SMM are diagnosed by excluding the presence of these serious symptomatic disorders, and by the absence of end-organ damage attributable to the underlying plasma cell proliferative process.
Monoclonal gammopathy of undetermined significance
Definition
MGUS is the most common plasma cell disorder and is the precursor of MM, and almost certainly WM and AL also. It is defined as a monoclonal serum immunoglobulin of no more than 3.0 g/dL, absence of hypercalcemia, renal insufficiency, anemia or bone lesions (CRAB), and fewer than 10% monoclonal plasma cells (PCs) in the bone marrow. The most common heavy chain type is immunoglobulin G (IgG) (70%), IgM (15%), IgA (12%), and biclonal gammopathy (3%).
Prevalence
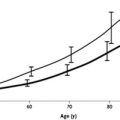
Earlier reports revealed that approximately 1.5% of people older than 50 years and 3% of the population older than 70 years in Sweden, the United States, and western France have an M protein without evidence of MM, WM, AL or a related disorder. In a population-based study conducted in Olmsted County, MN, 21,463 (77%) of the 28,038 residents 50 years of age and older were evaluated. The type of MGUS was IgG (69%), IgA (11%), IgM (17%), and biclonal (3%). The prevalence of MGUS was 3.2% (694 patients), with a prevalence of 5.3% in people 70 years of age or older, and in 8.9% in men older than 85 years. The age-adjusted rates were greater in men than in women at 4.0% versus 2.7% ( P <.01) ( Table 1 and Fig. 1 ). The size of the M protein was at least 2 g/dL in only 4.5% of MGUS cases, while the M protein was less than 1.5 g/dL in 80% of cases. Reduction of uninvolved immunoglobulins was present in 28% of the 447 patients who were tested. This study was performed in a predominantly white population. The annual incidence of MGUS in men is 120 cases per 100,000 population at the age of 50 years, increasing to 530 cases per 100,000 population at the age of 90 years.
| Age | Men | Women | Total |
|---|---|---|---|
| Number/Total Number (Percent) a | |||
| 50–59 y | 82/4038 (2.0) | 59/4335 (1.4) | 141/8373 (1.7) |
| 60–69 y | 105/2864 (3.7) | 73/3155 (2.3) | 178/6019 (3.0) |
| 70–79 y | 104/1858 (5.6) | 101/2650 (3.8) | 205/4508 (4.6) |
| ≥80 y | 59/709 (8.3) | 111/1854 (6.0) | 170/2563 (6.6) |
| Total | 350/9469 (3.7) b | 344/11,994 (2.9) b | 694/21,463 (3.2) b , c |
a The percentage was calculated as the number of patients with MGUS divided by the number who were tested.
b Prevalence was age-adjusted to the 2000 US total population as follows: men, 4.0% (95% CI, 3.5–4.4); women, 2.7% (95% CI, 2.4–3.0); and total, 3.2% (95% CI, 3.0–3.5).
c Prevalence was age- and sex-adjusted to the 2000 US total population.
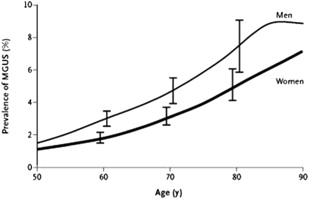
The prevalence of MGUS is approximately twice as high in blacks when compared with whites. In a study using samples from the National Health and Nutritional Examination Survey (NHANES), among 12,482 people age 50 years and older, the adjusted prevalence of MGUS was significantly higher in blacks (3.7%) compared with whites (2.3%) or Hispanics (1.8%), P = .001. The increased prevalence of MGUS is also seen in blacks from Africa; in a study of 917 men (50–74 years) from Ghana, the age-adjusted prevalence of MGUS was 5.84% (95% confidence interval [CI], 4.27–7.40). Compared with white men, the age-adjusted prevalence of MGUS was 1.97-fold (95% CI, 1.94–2.00) higher in Ghanaian men. These studies suggested a genetic predisposition, but a contributing effect of similar socioeconomic status could not be ruled out. In a subsequent study of 1000 black and 996 white women (age 40–79 years) of similar socioeconomic status for MGUS, the racial disparity persisted; the prevalence of MGUS was 3.9% in blacks versus 2.1% in whites. The presence of racial disparity after adjusting for socioeconomic status strongly suggests a genetic predisposition. In contrast to the increased prevalence seen in blacks, the prevalence of MGUS appears lower in Japan. In 1 study, 2.4% of 52,802 people 50 years of age or older in Nagasaki City, Japan, had MGUS.
First-degree relatives of people with MGUS have an increased risk of developing MGUS and related disorders. In a report of 911 relatives of 232 MM and 97 MGUS patients, there was a 2.0 relative risk (RR) among relatives of MM probands and an RR of 3.3 in MGUS probands.
Light Chain MGUS
Light chain MGUS is defined as the presence of an abnormal FLC ratio (normal 0.26–1.65), no monoclonal immunoglobulin heavy chain, an increased concentration of the involved light chain, absence of end-organ damage, and fewer than 10% monoclonal bone marrow PCs. In a study of 18,353 residents of southeastern Minnesota, light chain MGUS was present in 0.8% residents.
Etiology
The cause of MM is unknown, but both genetic and environmental factors are likely involved. In the study of Nagasaki atomic bomb survivors, people living within 1.5 km of the explosion had a 1.4-fold increase in MGUS compared with those living beyond 3.0 km. There was no difference in the prevalence of MGUS in older patients, but those no more than 20 years of age at the time of exposure had an increased prevalence of MGUS. In a report of 555 men from a well-documented prospective cohort of patients applying restricted-use pesticides, 6.8% had MGUS compared with 3.7% of men from Olmsted County, MN. The age-adjusted prevalence of MGUS was 1.9-fold greater among male pesticide workers.
Long-Term Outcome of MGUS
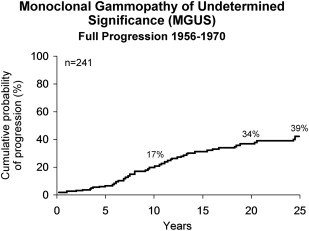
Two hundred and forty-one patients seen at the Mayo Clinic between 1956 and 1970 were followed for 3579 person years (median: 13.7 years, range 0–39 years). Fourteen (6%) were alive and had no substantial increase in M protein during a follow-up of 33 years. Twenty-seven percent developed MM, WM, AL, or a lymphoproliferative disorder. Patients in this group were followed for a median of 10.4 years (range 1–32 years) before diagnosis of the malignant lymphoplasmacytic proliferative disorder ( Table 2 ). The actuarial risk of progression was 17% at 10 years, 34% at 20 years, and 39% at 25 years (a rate of approximately 1.5% per year) ( Fig. 2 ). Sixty-nine percent of the 64 patients who progressed had MM. AL was found in 8 patients; WM occurred in 7 patients, and a lymphoproliferative disorder occurred in 5 patients.
| Number (%) of Patients | Interval to Disease (y) | ||
|---|---|---|---|
| Median | Range | ||
| Multiple myeloma | 44 (69) | 10.6 | 1–32 |
| Macroglobulinemia | 7 (11) | 10.3 | 4–16 |
| Amyloidosis | 8 (12) | 9.0 | 6–19 |
| Lymphoproliferative disease | 5 (8) | 8.0 | 4–19 |
| Total | 64 (100) | 10.4 | 1–32 |
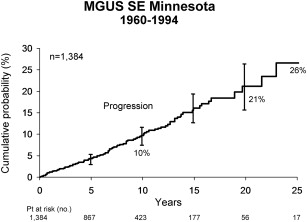
To eliminate a bias that occurs with referral populations, a separate population-based study of 1384 patients with MGUS from southeastern Minnesota was conducted. The median age at diagnosis was 72 years, in contrast to 64 years for the 241 referred patients. These patients were followed for a median of 15.4 years (range 0–35 years). MM, AL amyloidosis, lymphoma with IgM serum protein, WM, plasmacytoma, or chronic lymphocytic leukemia developed in 115 patients (8%) during follow-up ( Table 3 ). The risk of progression was 1% per year ( Fig. 3 ). It is important to emphasize that patients with MGUS continue to be at risk for progression as long as they live.
| Type of Progression | Observed Number of Patients | Expected Number of Patients a | Relative Risk (95% CI) |
|---|---|---|---|
| Multiple myeloma | 75 | 3.0 | 25.0 (20–32) |
| Lymphoma | 19 b | 7.8 | 2.4 (2–4) |
| Primary amyloidosis | 10 | 1.2 | 8.4 (4–16) |
| Macroglobulinemia | 7 | 0.2 | 46.0 (19–95) |
| Chronic lymphocytic leukemia | 3 c | 3.5 | 0.9 (0.2–3) |
| Plasmacytoma | 1 | 0.1 | 8.5 (0.2–47) |
| Total | 115 | 15.8 | 7.3 (6–9) |
a Expected numbers of cases were derived from the age- and sex-matched white population of the Surveillance, Epidemiology, and End Results program in Iowa, except for primary amyloidosis, for which data are from Kyle, et al.
b All 19 patients had serum IgM monoclonal protein. If the 30 patients with IgM, IgA, or IgG monoclonal protein and lymphoma were included, the relative risk would be 3.9 (95% CI, 2.6–5.5).
c All 3 patients had serum IgM monoclonal protein. If all 6 patients with IgM, IgA, or IgG monoclonal protein, and chronic lymphocytic leukemia were included, the relative risk would be 17 (95% CI, 0.6–3.7).
Risk Factors for Progression
Size and type of serum M protein
The size of the M protein is the single most important predictor of progression. At 20 years after recognition of MGUS, 14% with an initial M protein value of no more than 0.5 g/dL and 49% of those presenting with an M protein of 2.5 g/dL progressed. A serum M protein value of 1.5 g/dL had almost twice the risk of progression compared with a value of 0.5 g/dL. At 2.5 g/dL, the risk of progression was 4.6 times that of a value of 0.5 g/dL. A progressive increase in the size of M protein during the first year of follow-up is an important risk factor for progression.
Patients with an IgM or an IgA monoclonal protein have an increased risk of progression compared with patients with an IgG M protein. Varettoni and colleagues found that MGUS patients recognized more recently had a smaller M protein as well as a lower number of bone marrow PCs.
Bone marrow plasma cells
The presence of greater than 5% bone marrow PCs is an independent risk factor for progression. In another study, patients with bone marrow plasmacytosis of 10% to 30% had a malignant transformation rate of 37% compared with 6.8% in patients who had less than 10% PCs.
Serum FLC ratio
In a study of 1148 people with MGUS from southeastern Minnesota, an abnormal FLC ratio was detected in 33%. The risk of progression in patients with an abnormal FLC ratio was significantly higher (7.6%) compared with those with a normal ratio (hazard ratio 3.5; P <.001). This was independent of the size and type of the serum M protein.
Aberrant plasma cells
Perez-Persona reported that the presence of at least 95% aberrant PCs and DNA aneuploidy was associated with a significantly higher risk of progression to MM in a cohort of 407 MGUS and 93 SMM patients. Clonal PCs are discriminated from normal PCs by aberrant phenotypic expressions, typically consisting of simultaneous down-regulation of CD19 and CD45, with or without overexpression of CD56. If CD45 was positively expressed, lack of CD19 and/or bright CD56 staining allowed identification of clonal PCs.
Risk Stratification
The risk of progression of MGUS can be estimated using a simple model that uses the size and type of M protein and the serum FLC ratio. In a study of 1148 people with MGUS, patients with a serum M protein of at least 1.5 g/dL, non-IgG MGUS, and an abnormal serum FLC ratio had a risk of progression at 20 years of 58% (high-risk MGUS) compared with 37% for those with any 2 risk factors (high–intermediate risk), 21% when 1 risk factor was present (low–intermediate risk), and only 5% when none of the risk factors were found (low risk). If one considered the competing causes of death, the risk of progression was only 2% at 20 years in the low-risk group.
Differential Diagnosis
It is critical to differentiate MGUS from MM, Waldenström macroglobulinemia, and AL amyloidosis based on the presence or absence of end organ damage and extent of marrow involvement. A bone marrow aspirate and biopsy, as well as a radiographic bone survey, are indicated in all patients with an M protein value of at least 1.5 g/dL and in all patients with an unexplained abnormality in their hemoglobin, creatinine, or calcium levels. The authors have found the reduction of uninvolved immunoglobulins in serum or the presence of a monoclonal light chain in the urine (Bence Jones proteinuria) is of little help in distinguishing between MGUS and MM.
MM is often associated with circulating monoclonal PCs. The presence of cytogenetic abnormalities with fluorescence in situ hybridization (FISH) may be found in both MGUS and MM.
Secondary MGUS
Secondary MGUS is defined as the emergence of a new M protein different from the original clone during the course of MM. In 1 study, secondary MGUS was found in 6.6% of 1942 patients with MM at a median of 12 months after the diagnosis of MM. It most often occurs in patients who have had a stem cell transplant, and its presence is associated with better survival. More than 1 isotype occurred in approximately one-third of patients. Secondary MGUS is usually transient, with a median duration of 6 months; persistence beyond 1 year is associated with an adverse impact on survival.
Monoclonal gammopathy of renal significance
Monoclonal gammopathy of renal significance (MGRS) is a generic term used to denote patients with MGUS who have renal impairment that is felt to be related to the underlying monoclonal protein. The specific renal pathology may include membranoproliferative glomerulonephritis, C3 glomerulonephritis, and a variety of other renal disorders.
Idiopathic Bence Jones proteinuria
Bence Jones proteinuria is frequently present in patients with MM, Waldenström macroglobulinemia, and AL amyloidosis. Patients with Bence Jones protein in the urine without other evidence of lymphoplasmacytic disease are felt to have idiopathic Bence Jones proteinuria. These patients have FLCs that are secreted in quantities large enough to allow detection on urine protein electrophoresis. In this situation, idiopathic Bence Jones proteinuria may be an intermediate stage of disease between LC-MGUS and light chain MM and thus similar to SMM as an intermediate state between MGUS and MM. Some patients with idiopathic Bence Jones proteinuria may remain stable for many years without therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree