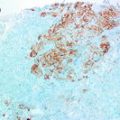
Fig. 6.1
Histologic grading of ductal carcinoma in situ (DCIS)
It should be noted that while interobservor variability in the classification of DCIS has improved significantly since the adoption of the newer systems, it is far from being completely eradicated. Nuclear grading is still a subjective interpretation and even the exact definition of comedonecrosis is under debate.
Prognosis
Despite the lack of extensive data on the natural progression of untreated DCIS, several large randomized clinical trials and cohort studies have identified few independent clinical and pathologic features associated with risk of disease recurrence or progression. Some of the factors associated with higher rates of local recurrence were younger age (≤40 years old), older age group (≥50 years old), symptomatic detection of DCIS, higher nuclear grade, solid or cribriform growth pattern, comedonecrosis, uncertain or involved margins or treatment with local excision alone [17–19].
Treatment modality has been shown to have significant influence on the recurrence rate, if not the overall survival. Until recently, mastectomy was the conventional treatment of DCIS [20]; however, with the success of breast conserving surgery/lumpectomy in invasive cancer, this conservative approach has been extended to DCIS as well. No randomized clinical studies comparing the efficacy of these two surgical options are currently available. On the other hand, radiotherapy (RT) has been shown to significantly decrease the rate of disease recurrence in clinical trials [17, 18, 21–26]. After lumpectomy alone, the risk of contralateral or ipsilateral disease recurrence ranges from 14 to 32 %, which is reduced by 40–50 % when paired with RT [17, 18, 21–26]. However, because RT does not seem to influence the overall survival rate, there is still a lack of consensus on the appropriate use of adjunct RT.
The use of improved DCIS classification, along with the identification of these risk factors has led to the development of prognostic systems such as the Van Nuys prognostic index (VNPI). The updated USC/VNPI stratifies DCIS patients according to age, size of the lesion, nuclear grade, and margin status and suggests differential treatment options according to the VPNI score [27, 28]. Although VPNI has been shown to be useful in a number of retrospective studies, it is yet to be validated in a prospective trial.
The current treatment protocol according to NCCN guidelines suggests lumpectomy ± radiation or mastectomy ± sentinel node biopsy. It was revised in 2008 to include lumpectomy alone as an option for those individuals with “low” risk, but does not specifically define that subset of patients. The guideline also recommends post-surgical treatment with tamoxifen in ER-positive DCIS, but does recognize that tamoxifen, like RT, reduces the risk of recurrence without improvement in overall survival rate [21, 25, 29]. The current guidelines demonstrate that despite the identification of several risk factors that are associated with higher disease recurrence, no systematically applied differential treatment protocols are currently in place for DCIS subtypes.
Tumorigenesis of DCIS
Several tumorigenesis pathways for DCIS have been proposed over the years. One model, first described by Wellings and colleagues in the 1970s, suggested flat epithelial atypia (FEA), ADH and DCIS as non-obligate precursor lesions to invasive ductal tumors [30–32]. Wellings further proposed that these ductal lesions, as well as lobular pre-malignant lesions, share a common progenitor in the terminal duct-lobular units (TDLUs) of the breast. Epidemiological, morphological, immunohistochemical and now molecular studies support this theory of evolutionary continuum between FEA, ADH, DCIS, and invasive ductal carcinoma (IDC)s, which is further detailed in the following section.
An alternative theory integrated benign epithelial proliferations such as UDH into this scheme, proposing progressive de-differentiation of UDH into malignancy [33]. Recent immunohistochemical and molecular studies however, have failed to demonstrate a clear relationship between UDH and other premalignant lesions. Rather, UDH appears to be more closely related to normal, non-proliferative breast epithelium and likely represents a distinct clinical entity unrelated to the pre-malignant lesions of the breast [34, 35].
The prevailing model of breast cancer progression has further refined Wellings’ original theory and now recognizes divergent pathways for low-and HG DCIS. First discovered in IDCs, it is now recognized that the same recurrent but differential molecular changes are largely recapitulated in the in situ lesions as well. For example, loss of 16q, the hallmark chromosomal abnormality of low-grade invasive carcinoma, is also observed in greater than 70 % of LG DCIS. In contrast, 16q loss is observed in only 30 % of HG DCIS. In addition, it has been recognized that low-grade DCIS are largely ER positive, whereas only a subset of the HG lesions express the hormone receptor. Furthermore, those HG DCIS that are ER positive tend to harbor the same chromosomal abnormalities typically associated with low-grade lesions. These findings, among others, suggest that while at least two distinct carcinogenetic pathways may exist, a subset of HG DCIS may indeed represent low-grade lesions that have progressively de-differentiated and possible points of intersection can be observed among the several breast cancer pathways (Fig. 6.2).


Fig. 6.2
Divergent pathways of low and high-grade breast cancer. LG pathway is characterized by positivity for ER/PR, Bcl-2 and low Ki-67 index. Chromosomes tend to be diploid or near-diploid with recurrent changes such as loss of 16q or gains of 1q or 16p. FEA and ADH are thought to be precursor lesions of the low-grade pathway and share similar expression of biomarkers and chromosomal abnormalities. Luminal A DCIS is the predominant molecular subtype seen in the low-grade pathway. HG pathway is characterized by negativity for ER/PR, positivity for p53 and high Ki-67 index, producing tumors with TN/basal-like or HER 2+ phenotype. These tumors also are frequently aneuploid and/or exhibit complex karyotype. MGA has been proposed as a possible precursor lesion for high-grade lesions with TN/basal-like phenotype. Overlap also exists between the LG and HG pathways. Some IG and HG DCIS show molecular features of both low and high-grade lesions, and may represent de-differentiated lesions of the LG pathway. (Figure adapted with permission from [36])
ER Estrogen receptor; PR progesterone receptor; LG low-grade; IG intermediate grade; HG high-grade; FEA flat epithelial atypia; ADH atypical ductal hyperplasia; TN triple negative; MGA microglandular adenosis; DCIS ductal carcinoma in situ; IDC invasive ductal carcinoma
FEA and ADH, in keeping with the theory of a common evolutionary pathway, share many of the immunohistochemical and molecular signatures of low-grade DCIS. Like the low-grade DCIS, FEA and ADH are generally positive for ER and PR but negative for HER 2 and basal cell markers. They have also been shown to share many of the recurrent genetic imbalances (e.g., loss of 16q) and are often found in coexistence with low-grade DCIS and invasive carcinomas. These immunophenotypic, molecular, and epidemiologic evidence demonstrates the close developmental relationship among these low-grade lesions and provide strong evidence that FEA and ADH are non-obligate, neoplastic precursors of the low-grade cancerous lesions of the breast.
It is yet unclear, however, what the precursor lesion of HG DCIS may be. The complex karyotype of HG DCIS intimates both the inherent genetic volatility of these lesions and the heterogeneity of its origin. A minority of the HG DCIS that harbor a similar genomic profile to the low-grade DCIS may represent de-differentiated lesions, while others may have arisen de novo. There exists, however, recent but limited evidence showing that a subset of microglandular adenosis (MGA) may be a precursor to triple negative (ER, PR, and HER2 negative) HG DCIS [36, 37]. MGA is a rare breast lesion composed of cytologically bland glands with an infiltrative growth pattern, largely considered to be a benign process. Its rarity however, in comparison to the incidence of HG DCIS, makes it an unlikely candidate as a common progenitor for HG lesions of the breast.
Chromosomal Aberrations of Low-and High-Grade DCIS
Low and HG DCIS, like their invasive counterparts, are characterized by distinct set of chromosomal aberrations. One of the hallmark chromosomal abnormalities seen in low-grade DCIS, as mentioned before, is the loss of 16q (70 %), as evidenced by multiple comparative genomic hybridization (CGH) and loss of heterozygosity (LOH) studies [38–40]. Other recurrent abnormalities associated with low-grade DCIS include loss of 17p and gain of 1q (>70 %) and 16p (>40 %). In addition, low-grade DCIS is characterized by diploid or near-diploid chromosome number and on average, have fewer total chromosomal abnormalities.
In contrast, HG lesions exhibit greater tendencies for aneuploidy, more complex karyotype and generally harbor multiple amplifications. Some of the specific and more frequently observed chromosomal abnormalities of HG DCIS include gains of 1q, 5p, 8q and losses of 8p, 11q, 13q, and 14q. The genomic profile of intermediate-grade DCIS, much like the nuclear and cytologic features that currently define the DCIS grading system, straddle the boundaries of the low-and HG lesions. Although intermediate-grade DCIS shared some of the distinct genetic signatures with the low-grade lesions, one study also found they had on average, higher number of genetic imbalances (5.5 vs. 2.5) compared to low-grade DCIS [38]. Table 6.1 is a detailed list of the recurrent genomic changes seen in low- and HG DCIS, as well as other proliferative breast lesions.
Table 6.1
Chromosomal aberrations of proliferative breast lesions
Lesion | Method | Losses | Gains | References |
|---|---|---|---|---|
UDH | LOH | 3p, 9p, 11p, 13q, 16q, 17q | – | [56] |
LOH | 13q, 14p, 16q, 17p, 17q | – | [57] | |
LOH | 9p, 11q, 11p, 13q, 14q, 17p, 17q | – | [39] | |
CGH | 13q, 16q | 12q, 16p, 20q | [58] | |
CGH | None | None | [59] | |
CGH | 1q | 16q, 17p, 21p | [60] | |
CGH | 13q | – | [61] | |
FEA | LOH | 3p, 11q, 16q, 17q | – | [62] |
LOH | 1p, 3p, 5q, 9p, 9q, 10q, 17p, 17q, 22q | – | [63] | |
CGH | 11q, 12q, 16q, 17p, 18p, 21, 22 | 7q, 11q, 15q, 16p, 17q, 19q | [64] | |
ADH | LOH | 16q, 17p | – | [65] |
LOH | 11p, 13q, 16q, 17p, 17q | – | [39] | |
LOH | 8p, 16q, 17q | – | [66] | |
LOH | 1q, 3p, 11p, 11q, 16q, 17p | – | [67] | |
CGH | 16q, 17p, 20p | 1q, 16q, 11q | [60] | |
CGH | 13q, 16q | 3p, 8q, 15q, 16p, 20q, 22q | [58] | |
CGH | 8p, 9p, 11q, 13q, 14q, 16q, 21q, Xp | 1p, 1q, 2q, 8q, 10p, 17q, 20q, 20q, 22q, Xp | [40] | |
LG-DCIS | LOH | 2p, 6q, 8p, 9p, 11p, 11q, 13q, 14q, 16q, 17p, 17q | – | [39] |
CGH | 11p, 14q, 16q, 17p | NA | [40] | |
CGH | 4q, 13q | 16p, 20q, 22q | [58] | |
CGH | 9p, 13q, 14q, 16q | 1q, 17q | [38] | |
IG-DCIS | CGH | 2q, 5q, 8p, 9q, 11q, 16q, 17p | 1q, 8q, 17q | [38] |
HG-DCIS | LOH | 2q, 6q, 8p, 9p, 11p, 11q, 13q, 14q, 16q, 17p, 17q | – | [39] |
CGH | 8p, 13q, 14q | 1q, 8p, 9q, 16q, 17q, 19q | [40] | |
CGH | 4q, 5q, 9p, 11q, 13q | 1q, 6p, 6q, 7q, 8q, 10q, 12q, 14q, 15q, 16p, 17q, 19q, 20q, 21q, 22q | [58] | |
CGH | 1p, 12q, 16q, 17q, 22q | 1p, 1q, 2q | [61] | |
CGH | 2q, 5q, 6q, 8p, 9p, 11q, 13q, 14q, 16q, 17p | 1q, 5p, 8q, 17q | [38] |
Immunophenotype of Low-and High-Grade DCIS
Immunohistochemical (IHC) studies of the transcriptomic profiles of DCIS also support the theory of divergent tumorigenesis. DCIS, like their invasive counterpart, can be divided into broad categories based on estrogen receptor (ER) positivity. ER is one of the most valuable and extensively studied biomarkers in the breast and is expressed in approximately 70 % of DCIS overall [41]. ER expression is strongly associated with low-grade in situ and invasive ductal lesions, with nearly 100 % of the low-grade DCIS expressing the hormone receptor. Molecular studies of IDC have also shown ER-positive and ER-negative tumors are intrinsically distinct entities with divergent pathologic and clinical features. ER expression, along with the presence of HER2 upregulation, is the major determinant in molecular classification of IDC.
Other biomarkers preferentially expressed in low-grade DCIS also include progesterone receptor (PR) and Bcl-2. PR, like ER, is a hormone receptor that is prognostic as well as predictive of response to hormone therapy [41]. Bcl-2 is an anti-apoptotic protein whose de-regulation has been associated with pathogenesis of breast cancer. The expressions of both proteins are positively associated with ER, and help define the immunoprofile of the low-grade ductal lesions of the breast.
Conversely, higher-grade lesions are negatively associated with ER, positively associated with [42–44] HER2 expression, p53 expression, and basal markers (CK5/6, EGFR) and display higher Ki-67 index. Somewhat paradoxically, HER2 amplification, which is typically associated with worse clinical outcome in invasive tumors, is seen with higher frequency in the in situ lesions (15–25 vs. 55–70 %). The reason for this disparity remains unclear, however. Some of the proposed mechanisms include: loss of HER2 expression as HER2-positive DCIS progresses to IDC; higher rates of disease progression in HER2-negative DCIS; and mammographic detection bias for HER2-positive DCIS due to their association with comedo necrosis and calcification, which may be more easily identified by imaging. Table 6.2 summarizes the expression rate of various biomarkers stratified by DCIS histologic grades.
Table 6.2
Expression of biomarkers in ductal carcinoma in situ (DCIS)
Biomarkers | Histologic grade of DCIS | |||
|---|---|---|---|---|
Low | Intermediate | High | Overall | |
ER | ||||
PR | ||||
AR | 36 % [44] | 51 % [44] | 26 % [44] | 37 % [44] |
Her2 | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||