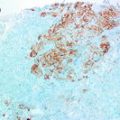
Fig. 8.1
Immunohistochemical staining of estrogen receptor showing granular/punctate brown nuclear staining in consistent with false positive pattern [55]
Predictive Value of Hormone Receptor Expression in Breast Cancer
A factor is predictive if it predicts the likelihood of responding to specific types of therapies both in the adjuvant and metastatic neoadjuvant setting. Hormone receptor expression in breast cancer is predictive of response to endocrine therapy that has been proven to be highly effective and appropriate for nearly all women with hormone receptor positive breast cancer, making such treatment the most widely prescribed therapy for patients with cancer in the world. For many years, tamoxifen taken for 5 years was the standard endocrine therapy for breast cancer. In a systematic review of randomised trials of adjuvant tamoxifen among women with early breast cancer (55 trials including 37,000 women) [40], it was demonstrated that in women with ER negative tumours (n = 8000) the overall effects of tamoxifen appeared to be small. In ER positive women (n = 30,000), adjuvant tamoxifen reduced the proportional recurrence during about 10 years of follow-up by 21, 29, and 47 %, for 1-year, 2-years and 5-years of treatment respectively. The corresponding proportional mortality reductions were 12, 17, and 26 %. The proportional mortality reductions were similar for women with node-positive and node-negative disease, but the absolute mortality reductions were greater in node-positive women [40]. More recently, patients who are postmenopausal also have been offered the option of taking an aromatase inhibitor (AI) as an alternative to tamoxifen or in sequence after tamoxifen [41].
Despite its advantages, the fact that patients who do not express ER do not benefit from hormone therapy added to an approximate 40 % of ER-positive BC patients who also do not respond to hormone therapy [42] provides important clinical context for researchers to explore more signalling pathways which may provide alternatives for novel targeted therapies. The understanding of the molecular biology and behaviour of breast cancer and a more robust classification of its different molecular subtypes is crucial in identifying newer therapeutic strategies. Using cDNA microarray, Jansen et al. [43] determined 81 genes for tamoxifen response among 46 ER-positive tumours from women with advanced ER-positive breast cancer after tamoxifen treatment. The genes were then shortlisted to 44 and validated on 66 tumours where they could predict tamoxifen response in 27 out of 35 cases. In another study, a new gene signature comprising 78 genes was identified using a set of 69 independent tumours from patients treated with tamoxifen [44]. The resistance to tamoxifen was also correctly predicted in 78 % of patients with relapse using a molecular signature of 36 genes, many of them were related to DNA proliferation and replication such as TK1 and CDC2 [45].
In patients with ER-positive disease, it is a major concern for oncologists as whether to add chemotherapy to the treatment plan or not. It has been reported that tumours which are ER positive are relatively resistant to chemotherapy compared to ER-negative ones with the absolute benefit from adjuvant chemotherapy significantly worse (7 % of ER-positive tumours vs. 22.8 % of ER-negative tumours) survived to 5 year disease free when receiving adjuvant chemotherapy [46, 47]. Tumours with high levels of both ER and PR are highly sensitive to endocrine therapy and the benefit of adjuvant chemotherapy is small in these cases, irrespective of menopausal status [48]. It remains unclear whether the lack of benefit from chemotherapy in these patients is due to an excellent outcome or due to genuine lack of biological effect. Recent data indicate that response to chemotherapy in ER positive breast cancer can be predicted using the commercially available Oncotype DX gene signature which is composed of a set of 16 genes with 5 reference genes using a polymerase chain reaction-type array [49].
Currently, the use of hormonal therapy in breast cancer is restricted to patients with tumours expressing ER and is prescribed in one of the following two clinical contexts: (1) in limited disease, it is either used in the adjuvant setting, i.e. after surgery to halt the growth of the metastatic cancer cells or as part of the neoadjuvant protocol to help shrink the tumour prior to surgery, OR (2) in the metastatic setting where surgical eradication of the disease in unlikely.
Although there is a gradient of increasing response to endocrine agents with increasing levels of ER, the gradient is skewed such that tumours expressing even very low numbers of positive cells (e.g. 1–10 %) show a significant benefit far above that of ER negative tumours, which are essentially unresponsive. The predictive value of quantitative PR expression for response to tamoxifen is less clear. Some authors showed that the benefit of endocrine adjuvant therapy is not affected by quantitative PR expression in ER positive breast cancer [50] while others have reported that the benefit of tamoxifen seems to be restricted to ER-positive breast cancers with a strong expression of PR (>75 %) [15]. Although some trials reported benefit of tamoxifen in ER negative breast cancer when PR is positive cases this may represent ER false negative assays [20, 29]. Tumours expressing both hormone receptor and HER2 pathways may be resistant to one or both targeted treatments due to interplay between these pathways and combination therapy may be more beneficial. The significance of the effect of adjuvant trastuzumab in ER-positive breast cancers is dependent on PR’s presence in HERA [51].
In menopausal women with ER positive breast cancer, there is a linear relationship between the quantitative expression of ER and the chance of responding to endocrine therapy, both for tamoxifen and oral AIs [52, 53]. Metastatic post-menopausal breast cancer patients diagnosed with ER positive breast cancer have an estimated clinical benefit from first line tamoxifen or AIs of 38–59 % [54].
In an update of guideline recommendations on adjuvant endocrine therapy for women with ER positive breast cancer by ASCO/CAP [41], it was recommended that:
1.
Pre- or perimenopausal women should be offered adjuvant endocrine therapy with Tamoxifen for an initial duration of 5 years. Additional therapy after 5 years should be based on menopausal status as follows:
A.
If women are pre- or perimenopausal, or if menopausal status is unknown or cannot be determined, they should be offered continued tamoxifen for a total duration of 10 years
B.
If women have become definitively postmenopausal, they should be offered continued tamoxifen for a total duration of 10 years or switching to up to 5 years of AIs, for a total duration of up to 10 years of adjuvant endocrine therapy.
2.
Women diagnosed with ER positive breast cancer who are postmenopausal should be offered adjuvant endocrine therapy with Tamoxifen for a duration of 10 years OR AIs for a duration of 5 years OR Tamoxifen for an initial duration of 5 years, then switching to an AI for up to 5 years, for a total duration of up to 10 years of adjuvant endocrine therapy OR Tamoxifen for a duration of 2–3 years and switching to an AI for up to 5 years, for a total duration of up to 7–8 years of adjuvant endocrine therapy.
3.
Women who are postmenopausal and are intolerant of either tamoxifen or an AI should be offered the alternative type of adjuvant endocrine therapy.
If women have received an AI but discontinued treatment at less than 5 years, they may be offered tamoxifen for a total of 5 years.
If women have received tamoxifen for 2–3 years, they should be offered switching to an AI for up to 5 years, for a total duration of up to 7–8 years of adjuvant endocrine therapy.
4.
Women who have received 5 years of tamoxifen as adjuvant endocrine therapy should be offered additional adjuvant endocrine treatment.
If women are postmenopausal, they should be offered continued tamoxifen for a total duration of 10 years or switching to up to 5 years AI, for a total duration of up to 10 years of adjuvant endocrine therapy.
If women are pre- or perimenopausal, or menopausal status cannot be ascertained, they should be offered 5 additional years of tamoxifen, for a total duration of 10 years of adjuvant endocrine therapy.
Conclusion
Hormone receptor is a strong predictor of endocrine therapy and should be assessed in all breast cancers including primary and recurrent. Hormone therapy is offered to all patients with ER positive breast cancer. Guideline recommendations for assessment of hormone receptor expression in breast cancer have been published. Adoption of these guidelines and implementation of various quality assurance methods should be encouraged to ensure high level of accuracy, precision and reproducibility of hormone receptor assays performance
References
1.
Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 2004;6(5):213–8.PubMedCentralCrossRefPubMed
2.
3.
4.
5.
Knight WA, et al. Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer Res. 1977;37(12):4669–71.PubMed
6.
Blamey RW, et al. Reading the prognosis of the individual with breast cancer. Eur J Cancer. 2007;43(10):1545–7.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree